Cu(I)-based metal-organic frameworks(铜铟金属有机框架)中多氧金属酸盐的电子吸附效应促进叠氮-炔烃 "点击 "反应
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
提高 Cu(I)位点的亲核性是提高 Cu(I)催化的叠氮-炔环加成反应(CuAAC)效率的基本策略。本研究采用原位溶热法合成了一种基于林奎斯特型聚氧化金属(POM)的金属有机框架[CuI4(W6O19)2(L)]-2H2O(NEAU-1)。单晶 X 射线衍射结果表明,NEAU-1 呈夹层结构,在间苯二酚[4]炔配体和铜(I)离子形成的二维层之间夹有 POM。NEAU-1 具有丰富的 Cu(I) 活性位点和较高的化学稳定性,使其成为 CuAAC 反应的有效异相催化剂。更重要的是,POMs 的存在有效降低了 Cu(I)位点周围的电子云密度,显著降低了形成铜乙酰化合物的能障,促进了后续的亲核反应。在 POMs 和 Cu(I) 的协同催化作用下,苄基叠氮化物和苯乙炔在 40 分钟内的转化率可达 99% 以上。这项工作提出了一种可持续的分子级策略,以提高 CuAAC 反应的活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
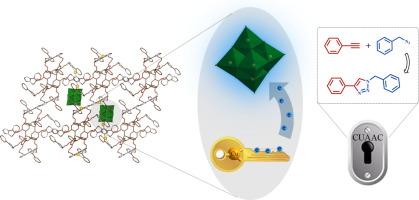
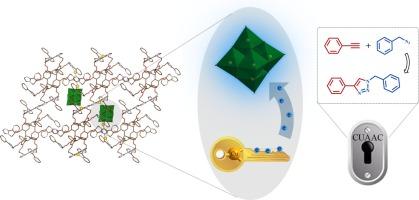
Electron-withdrawing effect of polyoxometalates in Cu(I)-based metal–organic frameworks for enhanced azide-alkyne “click” reaction
Boosting the nucleophilicity of Cu(I) sites is an essential strategy to enhance the efficiency of Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. In this work, a Lindquist-type polyoxometalate (POM)-based metal–organic framework, [CuI4(W6O19)2(L)]·2H2O (NEAU-1), was synthesized via an in-situ solvothermal method. Single-crystal X-ray diffraction results reveal that NEAU-1 exhibits a sandwich structure, with POMs intercalated between the two-dimensional layers formed by resorcin[4]arene ligands and Cu(I) ions. NEAU-1 possesses abundant Cu(I) active sites and high chemical stability, making it an effective heterogeneous catalyst for the CuAAC reaction. More importantly, the presence of POMs effectively reduces the electron cloud density around Cu(I) sites, significantly lowering the energy barrier for the formation of copper-acetylide compounds and facilitating subsequent nucleophilic reactions. The synergistic catalytic effect of POMs and Cu(I) can achieve a conversion rate of over 99 % for benzyl azide and phenylacetylene within 40 min. This work presents a sustainable molecular-level strategy to enhance the activity of the CuAAC reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


