铁光催化盖斯型加成中的配体转换效应
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
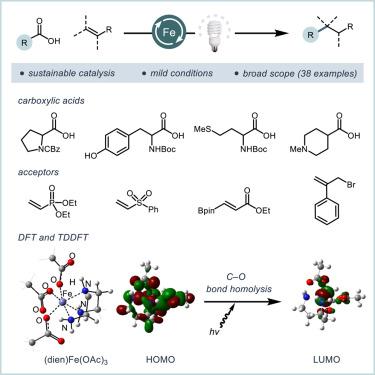
可见光促进的铁光催化是一种可持续的合成化学策略。然而,在许多转化过程中,通过配体-金属电荷转移事件进行的铁光催化反应仅限于简单的底物。该领域的一个突出挑战是如何选择具有一般反应活性的可调配体支架。在这项工作中,我们介绍了在铁促进的脱羧盖斯型加成中使用脂肪族胺作为配体的情况。与之前的光氧化报告不同,这种方法可以将底物与游离胺、醇和硼酸酯偶联。为了深入了解二乙烯三胺(L1)的作用,我们使用紫外可见光谱、热重分析和密度泛函理论对连接的铁盐进行了研究。这些研究支持了光活性八面体羧酸盐 (L1)Fe(OCOR)3 的形成。随着越来越多的光催化反应利用富土金属进行,这些配体的使用扩大了将铁应用于一系列广泛转化的可能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Transformative ligand effects in Fe-photocatalyzed Giese-type additions
Visible-light-promoted Fe photocatalysis is a sustainable strategy for synthetic chemistry. Yet, the adoption of Fe photocatalytic reactions proceeding through a ligand-to-metal charge transfer event has been limited to simple substrates in many transformations. An outstanding challenge in the field is the selection of tunable ligand scaffolds providing general reactivity. In this work, we describe the use of aliphatic amines as ligands in Fe-promoted decarboxylative Giese-type additions. Unlike prior photoredox reports, this method enables the coupling of substrates with free amines, alcohols, and a boronic ester. To gain insight into the role of diethylenetriamine (L1), ligated Fe salts were investigated using ultraviolet-visible spectroscopy, thermal gravimetric analysis, and density functional theory. These studies support the formation of a photoactive octahedral carboxylate (L1)Fe(OCOR)3. As an increasing number of photocatalytic reactions proceed using earth-abundant metals, the use of these ligands expands the possibility of applying Fe to a broad array of transformations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


