1,3-二烷基溴的电化学环丙烷化反应
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
本文展示了一种单取代和 1,1 二取代环丙烷的电化学合成方法。该方法从容易获得的 1,3-二烷基溴开始,将牺牲还原剂与具有成本效益的阴极和阳极材料结合在一起。改进后的方法无需使用分流电池,也无需使用危险或昂贵的电极,从而简化了该方案向连续流动系统的过渡。此外,还介绍了一种利用简单牺牲阳极的替代方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
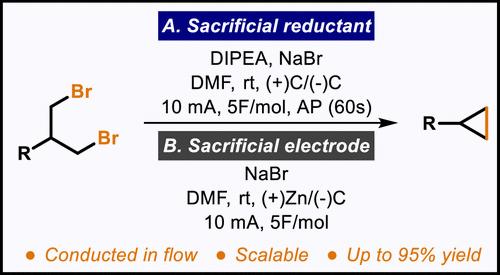
Electrochemical Cyclopropanation of 1,3-Dialkyl Bromides
An electrochemical synthesis of mono- and 1,1-disubstituted cyclopropanes is demonstrated. Starting from readily available 1,3-dialkyl bromides, this method hinges on the integration of a sacrificial reductant alongside cost-effective cathode and anode materials. The refined approach eliminates the necessity for a divided cell and the use of hazardous or costly electrodes, thereby streamlining the transition of this protocol to a continuous flow system. In addition, an alternative protocol that utilizes a simple sacrificial anode is also described.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


