开发可扩展的阿来替尼生产工艺,简明制备含吲哚的四环核心化合物
IF 3.5
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
摘要
阿来替尼(Alectinib,市场名为 Alecensa)是一种口服高效 ALK 抑制剂,用于治疗 ALK 阳性的非小细胞肺癌 (NSCLC)。本文介绍了从药物化学合成工艺到能够大规模供应高质量药物的工艺的演变过程。阿来替尼的一个结构特征是其含吲哚的四环核心,通过分子内还原环化和分子内弗里德尔-卡夫斯反应有效地构建了这一核心。此外,优化合成路线和条件的目的是抑制在下游工艺中难以清除的含有相同四环支架的杂质的形成。所建立的生产工艺可持续生产多公斤规模的阿来替尼,总产率通常为 29%,纯度超过 99.9%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
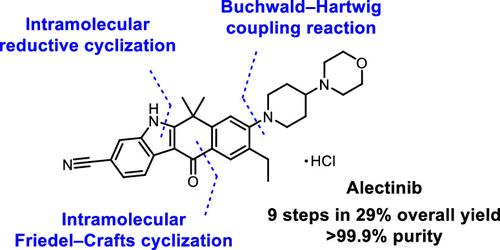
Development of a Scalable Manufacturing Process for Alectinib with a Concise Preparation of the Indole-Containing Tetracyclic Core
Alectinib (marketed as Alecensa) is an oral, highly potent ALK inhibitor for the treatment of ALK-positive, non–small-cell lung cancer (NSCLC). This paper describes the evolution from a medicinal chemistry synthetic process to a process enabling the scaled-up supply of a high-quality drug substance. A characteristic structural feature of alectinib is its indole-containing tetracyclic core, the construction of which was effectively achieved through intramolecular reductive cyclization and an intramolecular Friedel–Crafts reaction. Furthermore, the optimized synthetic route and conditions were designed to suppress the formation of impurities containing the same tetracyclic scaffold that are difficult to purge in downstream processes. The established manufacturing process could consistently produce alectinib on a multikilogram scale, typically with an overall yield of 29% and purity exceeding 99.9 area%.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


