通过自递胶束协同自噬细胞死亡和奥沙利铂诱导的免疫原性死亡,增强肿瘤免疫疗法。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
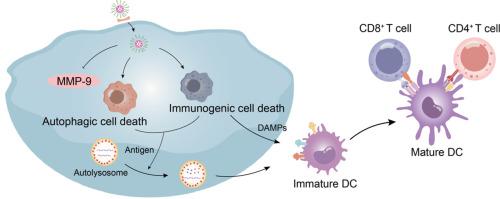
化疗已成为通过诱导免疫原性细胞死亡(ICD)激活细胞毒性T细胞反应的一种新兴策略,但由于肿瘤抗原呈递和T细胞激活不足,奥沙利铂(OXA)等化疗药物诱导的抗肿瘤免疫水平有限。诱导自噬细胞死亡(ACD)可促进肿瘤抗原的释放和树突状细胞的募集,从而加强抗肿瘤免疫反应。在这里,我们利用肿瘤靶向胶束同时激活ICD和ACD,以实现增强的抗肿瘤化疗免疫疗法。通过将 OXA 原药与生育酚琥珀酸酯(TOS)作为疏水段共轭,并进一步包裹自噬激活剂 SMER28,形成 TOPR/SMER28,它能以 c(RGDfK)特异性靶向肿瘤细胞上的αvβ3。细胞内化后,肿瘤细胞中高浓度的还原型谷胱甘肽(GSH)会从原药中释放出 OXA,引发 ICD 并释放相关分子模式(DAMPs)信号分子,从而刺激免疫。同时,SMER28 过度激活自噬,诱导自噬细胞死亡,进一步导致树突状细胞成熟,最终激活抗肿瘤免疫反应。在 4T1 肿瘤小鼠中,OXA 和 SMER28 的组合能有效抑制肿瘤生长并激活抗肿瘤免疫反应。肿瘤靶向胶束按需释放 OXA 和 SMER28,并通过协同 ICD 和 ACD 加强肿瘤化疗免疫疗法,为抗肿瘤免疫疗法提供了另一种选择。意义说明:化疗可诱导免疫原性细胞死亡(ICD),从而激活抗肿瘤免疫。然而,由于抗原呈递和 T 细胞活化水平较低,其疗效受到限制。为了加强 ICD 诱导的抗肿瘤免疫反应,我们首先将自噬细胞死亡(ACD)与 ICD 结合起来,配制了一种谷胱甘肽响应型奥沙利铂原药胶束,其中共同包裹了自噬激活剂 SMER28。SMER28 激活的自噬水平可增强抗原的释放和 APC 的招募,并最终增强 T 细胞介导的抗肿瘤免疫反应。我们提供了一种将自噬激活与化疗相结合以扩大抗肿瘤免疫效应的潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synergizing autophagic cell death and oxaliplatin-induced immunogenic death by a self-delivery micelle for enhanced tumor immunotherapy
Chemotherapy has become an emerging strategy to activate cytotoxic T cell responses by inducing immunogenic cell death (ICD), but the level of antitumor immunity induced by chemotherapeutic agents, such as oxaliplatin (OXA), is limited due to inadequate tumor antigen presentation and T cell activation. Inducing autophagic cell death (ACD) promotes the release of tumor antigen and the recruitment of dendritic cells, therefore strengthening antitumor immune responses. Here we simultaneously activate ICD and ACD with tumor targeting micelle to achieve enhanced antitumor chemo-immunotherapy. A self-delivery micelle is formulated by conjugating OXA prodrug with tocopherol succinate (TOS) as a hydrophobic segment and further encapsulates autophagy activator SMER28 to afford TOPR/SMER28, which specifically targets αvβ3 on tumor cells with c(RGDfK). Upon cellular internalization, OXA is released from the prodrug in response to the high concentration of reduced glutathione (GSH) in tumor cells, triggering ICD and releasing associated molecular patterns (DAMPs) signaling molecules to stimulate immunity. Meanwhile, SMER28 over-activates autophagy to induce autophagic cell death, which further leads to the maturation of dendritic cells and ultimately activates anti-tumor immune response. In the 4T1 tumor-bearing mice, the combination of OXA and SMER28 effectively inhibits tumor growth and activates antitumor immune responses. The tumor targeted micelle releases OXA and SMER28 in an on-demand profile and strengthens tumor chemo-immunotherapy by synergizing ICD and ACD, providing an alternative for antitumor immunotherapy.
Statement of significance
Chemotherapy induces immunogenic cell death (ICD) to activate anti-tumor immunity. However, the efficacy is limited by low levels of antigen presentation and T cell activation. To strengthen the antitumor immune responses induced by ICD, we first combine autophagic cell death (ACD) with ICD by formulating a glutathione-responsive oxaliplatin prodrug micelle co-encapsulating the autophagy activator SMER28. The activated autophagic level by SMER28 enhances the release of antigen and the recruitment of APCs, and ultimately bolsters T cell-mediated antitumor immune responses. We provide a potential strategy to amplify antitumor immune effects by combining autophagy activation with chemotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


