多功能纳米声纳增敏剂工程细菌克服肿瘤缺氧,增强声动力疗法。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
背景:超声触发的声动力疗法(SDT)具有较高的安全性和可接受性,已成为一种前景广阔的肿瘤治疗方法。然而,肿瘤致密的基质、缺氧的微环境以及治疗时机的不可预测性限制了超声增敏剂的有效性和抗肿瘤治疗效果。因此,为提高 SDT 的疗效,开发一种成像引导的缺氧肿瘤声纳增敏策略至关重要:在这项研究中,我们开发了一种生物杂交系统 CB@HPP,通过基因工程细菌表达过氧化氢酶(CB),并在这些细菌表面修饰纳米声纳敏化剂(HPP)。通过体外和体内实验评估了肿瘤缺氧缓解、肿瘤靶向性、生物相容性和抗肿瘤功效。此外,还评估了 CB@HPP 在体内和体外的光声(PA)、超声(US)和荧光(FL)成像效果:结果:静脉注射CB@HPP后,CB@HPP能够靶向肿瘤组织。CB@HPP具有高效的过氧化氢酶活性,能成功降解过氧化氢产生氧气。氧含量的增加缓解了肿瘤组织内的缺氧,从而增强了 CB@HPP 介导的抗肿瘤作用。此外,CB@HPP还具有FL/PA/US多模态成像功能,反映了CB@HPP在肿瘤中的聚集效应,并提示了治疗时机:结论:生物杂交系统 CB@HPP 能明显缓解肿瘤缺氧,多模态成像介导的产氧 SDT 能有效抑制肿瘤。该成像与治疗一体化的生物杂交系统为 SDT 提供了一种更高效、更具吸引力的癌症治疗策略:本研究开发了一种用于成像引导药物靶向递送和原位制氧的增敏 SDT 策略。我们设计了一种生物杂交系统 CB@HPP,它由具有催化制氧功能的工程菌和具有多模态成像能力的纳米声纳敏化剂杂交而成。CB@HPP 能明显缓解肿瘤缺氧,而多模态成像介导的产氧 SDT 能有效抑制肿瘤。这种集成成像和治疗的生物杂交系统为 SDT 提供了一种更高效、更具吸引力的癌症治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
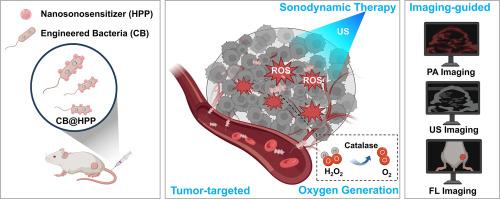
Multi-functional nanosonosensitizer-engineered bacteria to overcome tumor hypoxia for enhanced sonodynamic therapy
Background
Ultrasound-triggered sonodynamic therapy (SDT), with high safety and acceptance, has become a promising tumor treatment. However, the dense stroma, hypoxic microenvironment of tumor, and the unpredictable treatment timing limit the effectiveness of sonosensitizers and the antitumor therapeutic effect. Thus, it is crucial to develop an imaging-guided sensitization strategy for hypoxic tumor sonosensitization to improve the efficacy of SDT.
Methods
In this study, we developed a biohybrid system CB@HPP, which genetically engineered bacteria to express catalase (CB) and modified nanosonosensitizers (HPP) to the surface of these bacteria. Tumor hypoxia relief, tumor targeting, biocompatibility, and antitumor efficacy were evaluated through in vitro and in vivo experiments. In addition, the photoacoustic (PA), ultrasound (US), and fluorescence (FL) imaging effects of CB@HPP were evaluated in vivo and in vitro.
Results
After intravenous injection, CB@HPP was able to target tumor tissue. CB@HPP possessed efficient catalase activity and successfully degraded hydrogen peroxide to produce oxygen. Increased oxygen levels relief intratumoral hypoxia, thereby enhancing CB@HPP-mediated. In addition, CB@HPP showed FL/PA/US multimodal imaging capabilities, which reflects the aggregation effect of CB@HPP in the tumor and suggest the timing of treatment.
Conclusion
The biohybrid system CB@HPP significantly alleviates tumor hypoxia, and multimodal imaging-mediated oxygen-producing SDT effectively suppresses tumors. This integrated imaging and therapeutic biohybrid system provides a more efficient and attractive cancer treatment strategy for SDT.
Statement of significance
This study developed a sensitizing SDT strategy for imaging-guided drug-targeted delivery and in situ oxygen production. We designed a biohybrid system CB@HPP, which was hybridized by the engineered bacteria with catalytic oxygen production and nanosonosensitizer with multimodal imaging capability. CB@HPP significantly alleviates tumor hypoxia, and multimodal imaging-mediated oxygen-producing SDT effectively suppresses tumors. This integrated imaging and therapeutic biohybrid system provides a more efficient and attractive cancer treatment strategy for SDT.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


