作为氧泵的双活性纳米酶可缓解肿瘤缺氧并增强狙击性口腔鳞状细胞癌的光动力/近红外-II光热疗法。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
口腔鳞状细胞癌(OSCC)是头颈部最常见的恶性肿瘤,其治疗受到缺氧和供氧不足的限制。持续供氧结合光动力疗法(PDT)是解决这一问题的关键。在此,我们设计了一种具有双酶活性的海胆状 Au@Pt-Ce6-HN-1 纳米平台,作为 "氧气泵 "来缓解肿瘤缺氧,从而实现协同光动力/光热疗法(PTT)。在该设计中,光敏剂氯素 e6(Ce6)与 Au@Pt 纳米酶共价连接,用于光动力疗法。Au@Pt 纳米酶具有类似催化酶的活性,可持续分解肿瘤微环境中的 H2O2,提高 O2 水平,从而实现高效的光热效应。此外,Au@Pt 还能在近红外-II 光下执行 PTT 并提高氧气水平,从而进一步促进光动力疗法。Au@Pt 纳米酶还具有过氧化物酶样活性,可产生-OH,用于化学动力疗法(CDT)。此外,HN-1 还能引导 OSCC 的 "狙击 "方向,其高度特异性有利于 Au@Pt-Ce6-HN-1 在肿瘤部位的作用。Au@Pt-Ce6-HN-1 显示出明亮的荧光(FL)、强大的 CT 信号和光热成像能力,为后续的引导 PDT/PTT 奠定了基础。该纳米平台集持续制氧、肿瘤靶向和多模式成像等优势于一身,有望为治疗 OSCC 提供有价值的见解。意义声明:OSCC 的精确临床诊断和治疗具有挑战性。我们报告了一种具有双酶活性的海胆状 Au@Pt-Ce6-HN-1 纳米平台,该平台可作为 "氧气泵",引导治疗 OSCC 的光动力疗法(PDT)和光热疗法(PTT)。该纳米平台针对 OSCC 进行术前 CT 诊断,并为手术导航提供荧光可视化,显示出在临床癌症检测和手术指导方面的潜力。这种创新方法通过持续制氧、肿瘤靶向和多模式成像来解决 OSCC 缺氧问题并提高治疗效果,从而显著改善 OSCC 治疗的患者预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
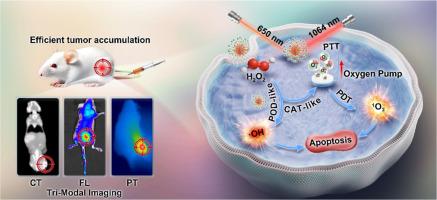
Dual-activity nanozyme as an oxygen pump to alleviate tumor hypoxia and enhance photodynamic/ NIR-II photothermal therapy for sniping oral squamous cell carcinoma
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor in the head and neck region, and its treatment is limited by hypoxia and inadequate oxygen supply. Continuous oxygen delivery combined with photodynamic therapy (PDT) is the key to addressing this issue. Here, a dual-enzyme activity sea urchin-like Au@Pt-Ce6-HN-1 nanoplatform was designed to serve as an "oxygen pump" to alleviate tumor hypoxia for synergistic photodynamic/photothermal therapy (PTT). In this design, the photosensitizer chlorin e6 (Ce6) is covalently linked to the Au@Pt nanozyme for PDT treatment. The Au@Pt nanozyme exhibits catalase-like activity, continuously decomposing H2O2 in the tumor microenvironment to enhance O2 levels, thereby achieving efficient PDT. Furthermore, Au@Pt can perform PTT and increase oxygen levels under NIR-II light to further promote PDT. The Au@Pt nanozyme also exhibits peroxidase-like activity, generating ·OH for chemodynamic therapy (CDT). Additionally, HN-1 guides the direction of "sniping" OSCC, and its high specificity benefits Au@Pt-Ce6-HN-1 at the tumor site. Au@Pt-Ce6-HN-1 exhibits bright fluorescence (FL), strong CT signal, and photothermal imaging capabilities, laying the foundation for subsequent guided PDT/PTT. This nanoplatform, which combines advantages such as continuous oxygen production, tumor targeting, and multimodal imaging, is expected to provide valuable insights into the treatment of OSCC.
Statement of significance
Accurate clinical diagnosis and treatment of OSCC are challenging. We report a dual-enzyme activity sea urchin-like Au@Pt-Ce6-HN-1 nanoplatform, serving as an "oxygen pump" to guide photodynamic therapy (PDT) and photothermal therapy (PTT) for OSCC. This nanoplatform targets OSCC for preoperative CT diagnosis and offers fluorescence visualization for surgical navigation, demonstrating potential in clinical cancer detection and surgery guidance. This innovative approach addresses OSCC hypoxia and enhances treatment efficacy through continuous oxygen production, tumor targeting, and multimodal imaging, significantly improving patient outcomes in OSCC treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


