基于数据驱动的微观结构模型,用于预测退变人体纤维环的圆周行为和失效。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
椎间盘纤维环的退化给理解和预测其力学行为带来了巨大挑战。在这篇文章中,我们提出了一种新的方法,通过对微观结构和退变进展的详细了解,准确预测退变人体椎间盘纤维环的力学特性。该框架的核心是一个基于连续体的全三维模型,该模型整合了水化状态和多尺度结构特征,包括蛋白多糖大分子和多层片状/片间软组织中不同层次的相互渗透的胶原纤维网络,能够承受形变引起的损伤。为了确保准确、全面地预测退变椎间盘环的机械行为,我们在椎间盘退变等级和个体年龄之间建立了数据驱动的相关性,这种相关性会影响椎间盘环成分的组成和机械完整性,同时考虑区域差异。该方法包括彻底识别与年龄和等级相关的模型输入演变,然后对模型的预测能力进行详细的定量评估,重点是圆周行为和失效。该模型成功地复制了实验数据,准确地捕捉了不同环形区域的刚度、横向响应(泊松比)和极限特性,同时还适应了年龄/等级关系的调节。正常退化和严重退化之间的降低率与实验数据相当吻合,内部区域的刚度降低幅度最大(34.63%),外部区域没有观察到明显变化。两个区域的破坏应力都大幅下降(内部区域为 49.86%,外部区域为 45.33%),而外部区域的破坏应变下降了 36.39%,内部区域下降了 24.74%。我们的研究结果表明,所提出的框架大大提高了从正常到严重退化状态的各种退化程度的环力学预测准确性。这种方法有望提高预测准确性,加深对椎间盘健康和损伤风险的了解,并为进一步研究退化对椎间盘完整性的影响奠定坚实的基础。意义说明:由于结构、生化和年龄相关因素的复杂相互作用,理解和预测退化的人体纤维环的机械行为仍然是一项重大挑战。本研究提出了一种基于微观结构的方法,通过整合水化状态、跨层次尺度的详细结构特征、变形诱发的损伤和失效,以及与年龄相关的变化和退化等级因素,来应对这一挑战。这种方法能够准确模拟各区域的环状力学,并将模型结果与现有数据进行全面比较,从而加强了其在捕捉退化效应方面的适用性。通过捕捉退化椎间盘中微观结构和机械行为之间错综复杂的相互作用,该模型为改进临床评估和指导未来椎间盘相关疾病的治疗策略奠定了坚实的基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
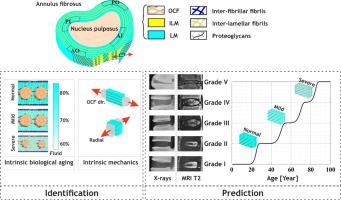
A data-driven microstructure-based model for predicting circumferential behavior and failure in degenerated human annulus fibrosus
The degeneration of the intervertebral disc annulus fibrosus poses significant challenges in understanding and predicting its mechanical behavior. In this article, we present a novel approach, enriched with detailed insights into microstructure and degeneration progression, to accurately predict the mechanics of the degenerated human annulus. Central to this framework is a fully three-dimensional continuum-based model that integrates hydration state and multiscale structural features, including proteoglycan macromolecules and interpenetrating collagen fibrillar networks across various hierarchical levels within the multi-layered lamellar/inter-lamellar soft tissue, capable of sustaining deformation-induced damage. To ensure accurate and comprehensive predictions of the degenerated annulus mechanical behavior, we establish a data-driven correlation between disc degeneration grade and individual age, which influences the composition and mechanical integrity of annulus constituents while accounting for regional variations. The methodology includes a thorough identification of age- and grade-related evolutions of model inputs, followed by a detailed quantitative evaluation of the model predictive capabilities, with a focus on circumferential behavior and failure. The model successfully replicates experimental data, accurately capturing stiffness, transverse response (Poisson's ratio), and ultimate properties across different annulus regions, while also accommodating the modulation of the age/grade relationship. The reduction rates between normal and severe degeneration align reasonably well with experimental data, with the inner region exhibiting the largest decrease in stiffness (34.63 %) and no significant change observed in the outer region. Failure stress drops considerably in both regions (49.86 % in the inner and 45.33 % in the outer), while failure strain decreases by 36.39 % in the outer and 24.74 % in the inner. Our findings demonstrate that the proposed framework significantly enhances the predictive accuracy of annulus mechanics across a spectrum of degeneration levels, from normal to severely degenerated states. This approach promises improved predictive accuracy, deeper insights into disc health and injury risk, and a robust foundation for further research on the impact of degeneration on disc integrity.
Statement of significance
Understanding and predicting the mechanical behavior of degenerated human annulus fibrosus remains a significant challenge due to the complex interplay of structural, biochemical, and age-related factors. This study presents a microstructure-based approach to address this challenge by integrating hydration state, detailed structural features across hierarchical scales, and deformation-induced damage and failure, alongside age-related changes and degeneration grade factors. This approach enables accurate simulations of annulus mechanics across regions, with model results thoroughly compared to available data, reinforcing its applicability in capturing degeneration effects. By capturing the intricate interactions between microstructure and mechanical behavior in degenerated discs, the model lays a strong foundation for improving clinical assessments and guiding future treatment strategies for disc-related conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


