膳食小球藻可减轻黄曲霉毒素 B1 对肉鸡的毒性:从毒理病理学、血液生化学和免疫学的角度看问题。
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
霉菌毒素是某些有毒真菌产生的次级代谢产物,对人类、鸟类和不同动物物种造成严重的健康问题,而绿藻(CV)是一种单细胞微藻,含有大量重要的营养成分。本研究计划评估在肉鸡日粮中添加黄曲霉毒素 B1(AFB1)所引起的毒理学、血液生化和免疫变化,以及通过 CV 对这些变化的缓解作用。为了开展这项研究,180 只一天龄的肉鸡被均匀地分为六(06)组,并施以不同的 AFB1(200 μg/kg )或 CV(0.5% 和 1.0%)组合,实验持续时间为 42 天。实验参数包括体重、采食量、相对内脏器官重量、大体和组织病理学检查、血液学参数(红细胞和白细胞计数、血细胞比容和血红蛋白)、血清生化分析(血清总蛋白、谷丙转氨酶、球蛋白、白蛋白、肌酐和尿素)、对绵羊红细胞的体液反应、对皮下注射植物血凝素-P的反应以及吞噬系统功能测试。实验结果证实,1.0% 的 CV 能有效缓解 AFB1 引起的研究参数的变化,而 0.5% 的 CV 与 AFB1 合用时,这种缓解是部分的。在这方面仍需进一步研究,以确定完全改善的 AFB1:CV 的确切比例。本文章由计算机程序翻译,如有差异,请以英文原文为准。
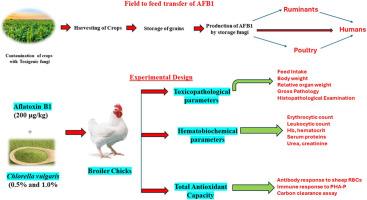
Dietary Chlorella vulgaris mitigates aflatoxin B1 toxicity in broiler chicken: Toxicopathological, hematobiochemical and immunological perspectives
Mycotoxins are the chemical substances, produced as the secondary metabolites of some toxigenic species of fungi which cause critical health issues in humans, birds and different animal species while Chlorella vulgaris (CV) is a unicellular microalga which contains plenty of important nutritional ingredients. This study was planned to evaluate the toxicopathological, hematobiochemical and immune changes incurred by dietary supplementation of aflatoxin B1 (AFB1) and their mitigation through CV in broilers. For this study to be conducted, 180 broiler birds of one day old were uniformly distributed into six (06) groups and administered various combinations of AFB1 (200 μg/kg) or CV (0.5 and 1.0%) and the duration of the experiment was 42 days. Parameters deliberated were body weight, feed intake, relative visceral organ weights, gross and histopathological examination, hematological parameters (erythrocytic and leukocytic count, hematocrit and hemoglobin), serum biochemical analysis (serum total proteins, ALT, globulin, albumin, creatinine and urea), humoral response against sheep RBCs, response to subcutaneous injection of phytohemagglutinin-P and phagocytic system function assay. The results of this experiment confirmed that 1.0% CV efficiently mitigated AFB1 induced alterations in the studied parameters while this mitigation was partial when 0.5% CV was used with AFB1. Further studies in this regard are still needed to investigate the exact AFB1:CV ratio responsible for complete amelioration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


