大戟科植物 Croton pulegiodorus Baill 叶子精油对小鼠急性口服毒性和遗传毒性评估。
IF 2.6
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
从 Croton pulegiodorus(巴豆)叶中提取的精油以其生物活性而闻名,但有关其毒性的数据却很有限。因此,本研究旨在评估一种 C. pulegiodorus 叶精油(CPLEO)的急性经口毒性和遗传毒性。采用气相色谱-质谱联用技术(GC-MS)对 CPLEO 进行了化学表征。体外试验验证了精油在小鼠红细胞中的溶血能力。接着,对雌性小鼠进行了急性口服毒性研究,CPLEO 的剂量分别为 2000、1000、500、250、100 和 50 毫克/千克。对未发生死亡的各组小鼠的血液学、生物化学和组织病理学指标进行了评估。此外,还记录了水和食物的相对消耗量以及动物及其器官的重量。最后,使用微核试验和彗星试验进行了遗传毒性分析。CPLEO 的提取率为 1.149%,其主要化合物为蛔虫醚(23.18%)、桉叶油醇(17.20%)、樟脑(14.20%)、对伞花烯(7.91%)、α-松油醇(4.69%)和乙酸异龙脑酯(4.57%)。CPLEO 仅在高浓度(185.5-1000 毫克/毫升)时才显示出溶血效应。它对小鼠的急性口服毒性为半数致死剂量(LD50)460.42 毫克/千克。CPLEO(50-250 毫克/千克)会导致血液和生化指标发生一些显著变化。组织病理学评估表明肝脏和肾脏发生了变化,但转氨酶、尿素和肌酐水平仍与阴性对照组相同。服用 CPLEO 会影响体重增加,减少水和食物的消耗量。最后,通过彗星和微核试验,CPLEO 不具有遗传毒性。这些结果突出表明,在评估CPLEO的生物活性时,需要注意选择剂量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
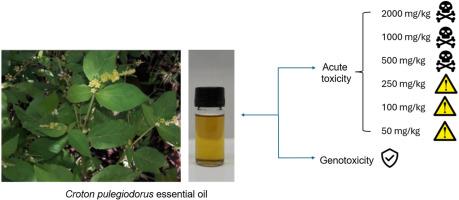
Acute oral toxicity and genotoxicity assessment of the essential oil from Croton pulegiodorus Baill (Euphorbiaceae) leaves in mice
Essential oils obtained from Croton pulegiodorus leaf are renowned for their biological activities; however, data on their toxicity are limited. Therefore, this study aimed to evaluate the acute oral toxicity and genotoxicity of a C. pulegiodorus leaf essential oil (CPLEO). Chemical characterization of CPLEO was conducted by gas chromatography coupled to mass spectrometry (GC-MS). In vitro assay was performed to verify the hemolytic capacity of the oil in mice erythrocytes. Next, an acute oral toxicity study was conducted on female mice at CPLEO doses of 2000, 1000, 500, 250, 100, and 50 mg/kg. Hematological, biochemical, and histopathological markers were assessed in mice from groups were no death occurred. Relative consumption of water and food and the weight of animals and their organs were also recorded. Finally, a genotoxicity analysis was performed using the micronucleus and comet assays. The extraction yield of CPLEO was 1.149% and its major compounds were ascaridole (23.18%), eucalyptol (17.20%), camphor (14.20%), p-cymene (7.91%), α-terpineol (4.69%), and isobornyl acetate (4.57%). CPLEO showed a hemolytic effect only at high concentrations (185.5–1000 mg/mL). It showed acute oral toxicity in mice with a LD50 of 460.42 mg/kg. CPLEO (50–250 mg/kg) caused some significant changes in hematological and biochemical parameters. Histopathological evaluation indicated alterations in liver and kidneys but transaminases, urea and creatinine levels remained like the negative control. CPLEO administration impaired weight gain and reduced water and food consumption. Finally, it was not genotoxic by both comet and micronucleus tests. The results highlight the need for attention when choosing doses to evaluate the bioactivities of CPLEO.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


