从Aglaia edulis (Roxb.) Wall.中提取的具有抗神经炎活性的黄烷以及相关黄烷的结构修订。
IF 3.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
从 Aglaia edulis (Roxb.) Wall 的树枝和树叶中分离出了八种环戊并[b]苯并呋喃(1、2、4 和 5-9)和八种环戊并[bc]苯并芘(3、10-16),其中包括一种经修订的化合物(4)和三种未被描述的化合物(1-3)。通过光谱分析、核磁共振和 ECD 计算,确定了这些化合物的结构。此外,根据 13C NMR 计算和 DP4+ 统计分析的结果,通过汇总已知化合物的化学位移数据,建立了区分环戊并[bc]苯并呋喃和环戊并[b]苯并呋喃结构的经验准则。该指南有助于对之前报告的三种类似物(R-1、R-2 和 R-3)的结构进行修正。生物学实验表明,环戊并[b]苯并呋喃黄烷(2 和 4-8)能显著抑制 LPS 诱导的 BV-2 细胞的 NO 生成,IC50 值为 0.002 至 0.05 μM。本文章由计算机程序翻译,如有差异,请以英文原文为准。
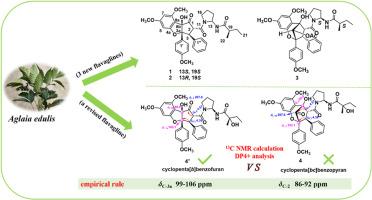
Flavaglines with anti-neuroinflammatory activity from Aglaia edulis (Roxb.) Wall. and structure revision of related flavaglines
Eight cyclopenta[b]benzofurans (1, 2, 4, and 5-9) and eight cyclopenta[bc]benzopyrans (3, 10–16), including a revised (4) and three undescribed compounds (1–3), were isolated from the twigs and leaves of Aglaia edulis (Roxb.) Wall. Their structures were determined by a combination of spectral analysis in conjunction with NMR and ECD calculations. Moreover, based on the findings from 13C NMR calculations and DP4+ statistical analysis, an empirical guideline was established to differentiate the structures of cyclopenta[bc]benzopyrans and cyclopenta[b]benzofurans by aggregating chemical shift data from known compounds. This guideline facilitated the proposal of structural revisions for three previously reported analogs (R-1, R-2, R-3). Biological assay indicated that cyclopenta[b]benzofuran flavalines (2, and 4–8) could significantly inhibit NO production in LPS-induced BV-2 cells with IC50 values from 0.002 to 0.05 μM.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytochemistry
生物-植物科学
CiteScore
6.40
自引率
7.90%
发文量
443
审稿时长
39 days
期刊介绍:
Phytochemistry is a leading international journal publishing studies of plant chemistry, biochemistry, molecular biology and genetics, structure and bioactivities of phytochemicals, including ''-omics'' and bioinformatics/computational biology approaches. Phytochemistry is a primary source for papers dealing with phytochemicals, especially reports concerning their biosynthesis, regulation, and biological properties both in planta and as bioactive principles. Articles are published online as soon as possible as Articles-in-Press and in 12 volumes per year. Occasional topic-focussed special issues are published composed of papers from invited authors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


