对寄主尸体的破坏模拟了清道夫的作用,对昆虫病原线虫物种的适应性产生了不同的影响。
IF 3.6
3区 生物学
Q1 ZOOLOGY
引用次数: 0
摘要
被昆虫病原线虫(EPN)感染的昆虫尸体会通过化学和其他手段来抵御食腐动物。尽管有这些防御措施,昆虫尸体仍有可能在被排斥前被咬伤。在这项研究中,我们调查了尸体角质层受损对在其内部发育的线虫(Heterorhabditis downesi 或 Steinernema feltiae)生存能力的影响。我们首先在野外量化了清道夫对受EPN感染的林奈瘿蚊尸体的破坏程度,并在实验室分别量化了蟋蟀(Gryllus bimaculatus De Geer)的破坏程度。在野外和实验室中,与冷冻杀死的对照组相比,受 EPN 感染的尸体受到的损害较小,损害主要是角质层的小损伤。在进一步的实验中,在受感染的尸体死后不久,通过刺穿表皮 0、1、3 或 5 次,并在潮湿(相对湿度为 100%)或干燥(相对湿度为 60-70%)的条件下培养,来模拟清扫损伤。损伤程度越大,尸体的水分损失越多(按体重损失估算),而在干燥条件下,这种损失会加剧。受 H. downesi 感染的尸体中出现的感染性幼体(IJs)数量因损坏而显著减少,尤其是在干燥条件下。此外,随着损害程度的增加,新出现的感染幼体的数量也在逐渐减少。该物种的 IJ 数量与水分损失呈负相关,这表明适应性的降低是由干燥引起的。对于 S. feltiae 而言,损害对 IJ 数量的影响较小,而大小则不受影响。水分损失无法解释数量减少的原因,这表明对于 S. feltiae 而言,当角质层受损时,除干燥外的其他因素(也许是与机会微生物的竞争)也会影响线虫。H. downesi比 S. feltiae更容易受到清道夫对宿主尸体的破坏,这可能是由于它在宿主体内的发育时间更长,导致暴露在破坏性条件下的时间更长。总之,食腐动物模拟咬噬的损害会影响 EPN 的适应性,影响程度取决于线虫种类、环境条件和损害程度。这些发现对在受感染尸体中实地应用 EPN 的成功与否有影响。本文章由计算机程序翻译,如有差异,请以英文原文为准。
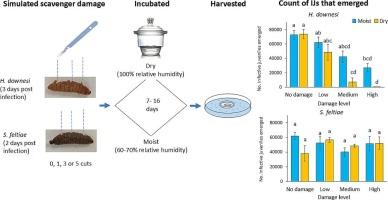
Damage to the host cadaver, simulating the effects of scavenging, differentially affects fitness of entomopathogenic nematode species
Insect cadavers infected by entomopathogenic nematodes (EPN) are defended against scavengers by chemical mechanisms and other means. Despite these defences, the cadaver may be bitten before being rejected. In this study, we investigated the effect of damage to the cadaver cuticle on the fitness of nematodes (Heterorhabditis downesi Stock, Griffin & Burnell or Steinernema feltiae Filipjev) developing inside. We first quantified the severity of scavenger damage to EPN-infected Galleria mellonella Linnaeus cadavers in the field, and separately, with crickets (Gryllus bimaculatus De Geer) in the laboratory. In both field and laboratory, EPN-infected cadavers suffered less damage than freeze-killed controls, and damage consisted mainly of small lesions to the cuticle. In further experiments, scavenging damage was simulated shortly after death of infected cadavers by piercing the cuticle 0, 1, 3 or 5 times and incubating in moist (100% relative humidity (RH)) or dry (60–70% RH) conditions. The greater the level of damage, the greater the loss of moisture from the cadaver (estimated by weight loss), and this was exacerbated in dry conditions. The number of infective juveniles (IJs) emerging from H. downesi-infected cadavers was significantly reduced by damage, especially in dry conditions. In addition, emerging IJs were progressively smaller with increasing damage. For this species, the number of IJs was negatively correlated with moisture loss, indicating that the reduction in fitness was mediated by desiccation. For S. feltiae, damage impacted IJ number to a lesser extent and size was not affected. The reduction in numbers was not explained by moisture loss, indicating that for S. feltiae, some factor other than desiccation (perhaps competition with opportunistic microbes) impacts the nematodes when the cuticle is damaged. The greater vulnerability of H. downesi, compared to S. feltiae, to scavenger damage to the host cadaver may be due to its longer developmental time in the host resulting in longer exposure to damaging conditions. In conclusion, damage simulating biting by scavengers impacts the fitness of EPN, with the effect depending on nematode species, environmental conditions and the extent of damage. These findings have implications for the success of field application of EPN in infected cadavers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
5.90%
发文量
94
审稿时长
1 months
期刊介绍:
The Journal of Invertebrate Pathology presents original research articles and notes on the induction and pathogenesis of diseases of invertebrates, including the suppression of diseases in beneficial species, and the use of diseases in controlling undesirable species. In addition, the journal publishes the results of physiological, morphological, genetic, immunological and ecological studies as related to the etiologic agents of diseases of invertebrates.
The Journal of Invertebrate Pathology is the adopted journal of the Society for Invertebrate Pathology, and is available to SIP members at a special reduced price.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


