艾滋病毒融合抑制剂 LP-98 负载微球在猕猴体内的长效释放和抗病毒疗效。
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
不坚持抗逆转录病毒治疗是有效控制艾滋病进展、降低传播率和死亡率的关键障碍。要解决用户依赖性给药的临床弊端并减少用药频率,一种有前途的策略是开发长效抗逆转录病毒药物。在这项研究中,我们制备了含有脂肽 LP-98 (LP-98-MS)的聚乳酸丙烯酸甲酯(PLGA)微球(MS)。我们的研究结果表明,在感染 SHIV 的猕猴体内单剂量注射 LP-98-MS 后,药物可持续、逐渐释放,抗病毒效果可维持至少 28 天。值得注意的是,单次注射 LP-98-MS 可提供超过 28 天的持续释放,从而为猕猴提供高水平的暴露前预防(PrEP),甚至在反复暴露于阴道内和直肠内 SHIV 挑战时提供完全保护。总体而言,LP-98-MS 在减少用药次数方面具有巨大潜力,并显示出良好的进一步开发前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
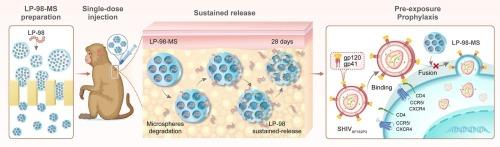
Prolonged release and antiviral efficacy of HIV fusion inhibitor LP-98-loaded microspheres in rhesus macaques
Non-adherence to antiretroviral treatment is a critical obstacle to effectively managing the progression of AIDS and reducing transmission and mortality rates. A promising strategy to address the clinical disadvantages of user-dependent dosing and decrease medication frequency is the development of long-acting antiretrovirals. In this study, we fabricated PLGA microspheres (MS) incorporating the lipopeptide LP-98 (LP-98-MS), which has previously exhibited potent anti-HIV efficacy. Our findings demonstrate that a single-dose injection of LP-98-MS in SHIV-infected rhesus macaques resulted in sustained and gradual release, maintaining antiviral effects at least 28 days. Notably, a single administration of LP-98-MS provided more than 28 days of sustained release, resulting in high-level pre-exposure prophylaxis (PrEP) for rhesus macaques, even providing complete protection when exposed to repeated intravaginal and intrarectal SHIV challenges. Overall, LP-98-MS holds significant potential in reducing medication frequency and shows promising prospects for further development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


