基于微粒子和纳米粒子的流感疫苗。
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
流感感染是每年全球范围内的公共卫生问题,有可能成为下一次大流行病。接种疫苗是预防未来流感爆发的最有效策略,然而,由于病毒抗原的漂移和转变,流感疫苗每年都需要重新配置以提供保护。由于需要更有效的流感疫苗,因此有必要重新研究提高现有疫苗免疫原性和广泛反应性的策略。在此,我们回顾了目前美国市场上获批的疫苗及其生产平台。我们讨论了开发广泛反应性疫苗的不同方法,并回顾了正在研究的可能用于未来流感疫苗配方的佐剂系统。我们还回顾了参与实现保护性免疫反应的免疫系统的主要组成部分,以及它们如何参与颗粒在全身和粘膜中的运输。最后,我们根据微粒和纳米颗粒的理化特性,对一些可用作肠外和粘膜疫苗接种递送系统的潜在微粒和纳米颗粒平台进行了描述和分类。本文章由计算机程序翻译,如有差异,请以英文原文为准。
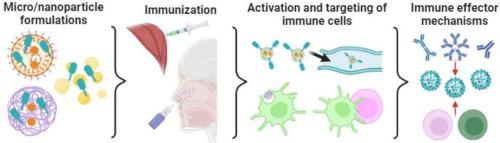
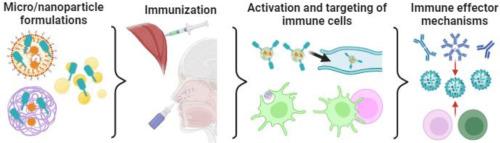
Microparticle and nanoparticle-based influenza vaccines
Influenza infections are a health public problem worldwide every year with the potential to become the next pandemic. Vaccination is the most effective strategy to prevent future influenza outbreaks, however, influenza vaccines need to be reformulated each year to provide protection due to viral antigenic drift and shift. As more efficient influenza vaccines are needed, it is relevant to recapitulate strategies to improve the immunogenicity and broad reactivity of the current vaccines. Here, we review the current approved vaccines in the U.S. market and the platform used for their production. We discuss the different approaches to develop a broadly reactive vaccine as well as reviewing the adjuvant systems that are under study for being potentially included in future influenza vaccine formulations. The main components of the immune system involved in achieving a protective immune response are reviewed and how they participate in the trafficking of particles systemically and in the mucosa. Finally, we describe and classify, according to their physicochemical properties, some of the potential micro and nano-particulate platforms that can be used as delivery systems for parenteral and mucosal vaccinations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


