从 Curvularia moringae 真菌培养物中分离出的多酮类化合物和生物碱的化学结构。
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从真菌 Curvularia moringae JKYM-KR4 中分离出了七种新的多酮类化合物[moringols I-VII (1-7)]、一种新的生物碱[moringamine I (8)]和七种已知化合物(9-15)。新化合物的平面化学结构和相对构型是通过高分辨率质谱、一维和二维核磁共振光谱以及利用计算的 13C 核磁共振化学位移进行 DP4+ 分析而阐明的。对于吗啉醇 I 和 II(1 和 2),其平面化学结构和相对构型已通过 X 射线晶体学得到证实。1-6 和 8 的绝对构型是通过 ECD 计算确定的。在分离出的化合物中,松油醇(14)能中度抑制 HT-29 细胞的增殖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
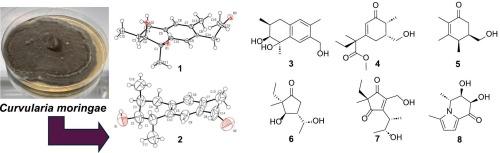
Chemical structures of polyketides and alkaloids isolated from a culture of fungus Curvularia moringae
Seven new polyketides [moringols I–VII (1–7)], a new alkaloid [moringamine I (8)], and seven known compounds (9–15) were isolated from the fungus Curvularia moringae JKYM-KR4. The planar chemical structures and relative configurations of the new compounds were elucidated by high-resolution mass spectrometry, 1D and 2D NMR spectroscopy, and DP4+ analysis using the calculated 13C NMR chemical shifts. For moringols I and II (1 and 2), the planar chemical structures and relative configurations were confirmed using X-ray crystallography. The absolute configurations of 1–6 and 8 were determined by ECD calculations. Among the isolated compounds, terpestacin (14) moderately inhibited the proliferation of HT-29 cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


