利用短期化学应力研究评估初始高分子量水平对单克隆抗体颗粒形成动力学的影响。
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
蛋白质制剂可能会形成大小从纳米到毫米不等的蛋白质颗粒。通过加速降解研究等方法监测蛋白质颗粒形成的动力学过程,是了解和评估颗粒数量的速度和进展的一种尝试。至于蛋白质溶液中高分子量(HMW)物种的初始水平或初始 HMW 水平(IHL)是否会影响蛋白质颗粒形成的传播,进而影响蛋白质的储存稳定性,目前所知甚少。在这项研究中,我们建立了一种通过热应力生成不同 IHL 的蛋白质溶液的方法。我们使用尺寸排阻色谱法(SEC)和亚可见颗粒分析法对两种单克隆抗体(mAb-A 和 mAb-B)在 40 °C 下不同 IHL 的 16 周热稳定性进行了评估。我们用盐酸胍(GuaHCl)在室温下进行了 300 分钟的等温压力研究,以评估通过 SEC 分析的高分子量的形成情况。应用芬克-瓦茨基(F-W)两步成核模型,我们可以用数学方法描述 HMW 形成的动力学,并提取这一过程的动力学参数。对于 mAb-A,IHL 对单体损失率的影响微乎其微;相反,mAb-A 在 40 °C 时会出现碎裂,这与 IHL 无关。然而,当 IHL 临界值超过 ≥ 7 % 时,现有的三聚体/四聚体会在 40 °C 时转化为高阶低聚物,而在 IHL 较低时则不会出现这种情况。与此相反,mAb-B 在超过 ≥ 4 % IHL 的阈值时表现出更高的 HMW 形成率,这反映在 40 °C 时的单体衰减率和化学应力研究的 F-W 动力学参数中。该案例研究表明,HMW 的初始水平对 HMW 的形成过程有不同的影响。在一个例子中,随着 IHL 的升高,HMW 的形成速度明显加快。相反,在另一个例子中,IHL 只对 HMW 的形成产生轻微影响。此外,我们的短期化学应力研究结果与在 40 °C 下进行的经典储存稳定性研究结果一致,该研究评估了不同的 IHL。对 HMW 形成动力学的分析将加深我们对蛋白质颗粒形成过程的理解,促进生物治疗药物的配方开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
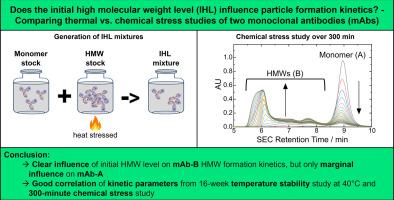
Evaluating the influence of the initial high molecular weight level on monoclonal antibody particle formation kinetics using a short-term chemical stress study
Protein formulations may form proteinaceous particles that vary in size from nanometers to millimeters. Monitoring the kinetics of protein particle formation, e.g., through accelerated degradation studies, is an attempt to understand and assess the rate and progression of particle populations. Little is known about whether the initial level of high molecular weight (HMW) species, or initial HMW level (IHL), of a protein solution influences the propagation of protein particle formation, and thus affects the storage stability of proteins.
In this study, we have established a method to generate protein solutions of different IHLs by thermal stress. We have evaluated a 16-week thermal stability study at 40 °C of two monoclonal antibodies (mAb-A and mAb-B) at different IHLs using size exclusion chromatography (SEC) and sub-visible particle analysis. We have performed an isothermal stress study with guanidinium hydrochloride (GuaHCl) at room temperature for 300-min to evaluate the formation of HMWs analysed by SEC. The application of the Finke-Watzky (F-W) two-step nucleation model allowed us to mathematically describe the kinetics of HMW formation and to extract kinetic parameters of this process.
For mAb-A, the IHLs had a marginal influence on the loss of monomer rate; instead, mAb-A exhibited fragmentation at 40 °C, which was independent of the IHL. Nevertheless, above a threshold of ≥ 7 % IHL, existing trimers/tetramers undergo conversion into higher-order oligomers at 40 °C, which is not observed at lower IHLs. In contrast, mAb-B exhibited an increased HMW formation rate above a threshold of ≥ 4 % IHL, which was reflected in the monomer decay rates at 40 °C and the F-W kinetic parameters of the chemical stress study.
This case study shows that the initial level of HMWs exerts a differential influence on the progression of HMW formation. In one instance, there is a discernible acceleration in the formation of HMWs with rising IHLs. Conversely, in another example, the IHL exerts only a slight influence on HMW formation. Moreover, the results of our short-term chemical stress study are in accordance with those of a classical storage stability study conducted at 40 °C, which evaluated different IHLs. The analysis of HMW formation kinetics will enhance our understanding of the protein particle formation process and facilitate the formulation development of biotherapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


