肾细胞癌胰腺转移的不同分子特征和共同的药物弱点。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
透明细胞肾细胞癌(ccRCC)是胰腺转移瘤(PM)最常见的起源。有报道称,透明细胞肾细胞癌(ccRCC)存在不同的基因组畸变、良好的预后、高血管生成以及对酪氨酸激酶抑制剂(TKI)的敏感性。然而,迄今为止还没有进行过功能或单细胞研究。我们招募了五名PM-ccRCC患者,研究了他们的患者衍生细胞(PDCs)的基因组、单细胞转录组和药物敏感性特征。PM描述了预期的和新的基因组改变。此外,转录组学与原发性和转移性 ccRCC 都有所不同,PI3K/mTOR 和血管生成通路上调,这与临床观察结果相吻合。通路层面的数据整合显示,转录组学最能解释药物敏感性。因此,PM-ccRCC PDCs对许多PI3K/mTOR抑制剂具有共同的敏感性。总之,我们在PM-ccRCC中发现了不同的基因组和转录组特征,突出了转录组学在解释药物敏感性方面的优势,并鼓励在PM-ccRCC中使用TKIs和PI3K/mTOR抑制剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。
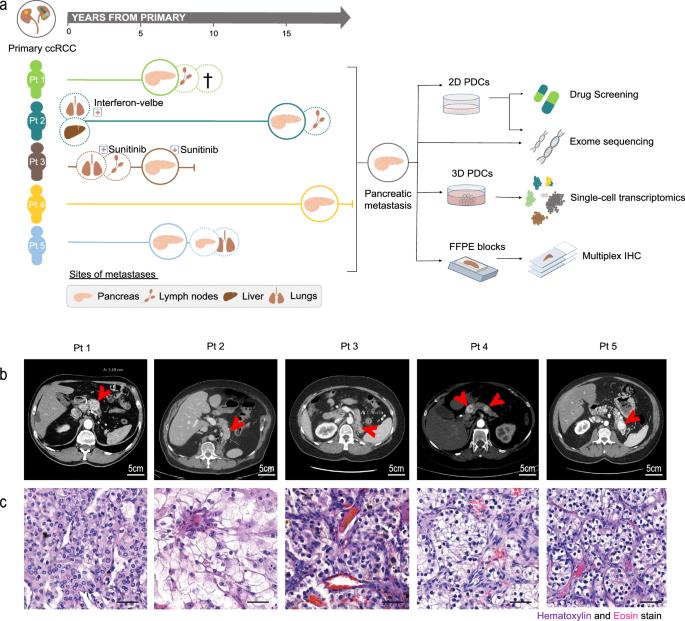
Distinct molecular profiles and shared drug vulnerabilities in pancreatic metastases of renal cell carcinoma
Clear-cell renal cell carcinoma (ccRCC) is the most common origin of pancreatic metastases (PM). Distinct genomic aberrations, favorable prognosis, and clinical observations on high angiogenesis, and succeeding tyrosine kinase inhibitor (TKI) sensitivity have been reported in PM-ccRCC. However, no functional or single-cell studies have been conducted thus far. We recruited five PM-ccRCC patients and investigated the genomic, single-cell transcriptomic, and drug sensitivity profiles of their patient-derived cells (PDCs). The PM depicted both expected and novel genomic alterations. Further, the transcriptomics differed from both primary and metastatic ccRCC, with upregulations of the PI3K/mTOR and – supporting the clinical observations – angiogenesis pathways. Data integration at pathway level showed that transcriptomics explained drug sensitivities the best. Accordingly, PM-ccRCC PDCs shared sensitivity to many PI3K/mTOR inhibitors. Altogether, we show distinct genomic and transcriptomic signatures in PM-ccRCC, highlight the superiority of transcriptomics in interpreting drug sensitivities, and encourage the use of TKIs and PI3K/mTOR inhibitors in PM-ccRCC. Functional precision medicine approach reveals genomic and transcriptomic aberrations that distinguish pancreatic metastases from other types of ccRCC metastases and suggest potential therapeutic targets at the individual level.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


