来自海洋硝化甘油菌的 4-羟基丁酰-CoA 合成酶(ADP 形成)的晶体结构。
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
摘要
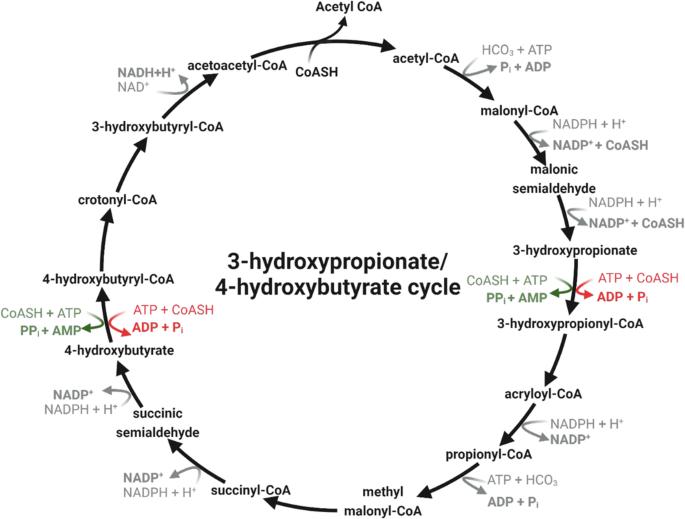
来自氨氧化潮虫的 3-hydroxypropionate/4-hydroxybutyrate (3HP/4HB) 循环目前被认为是能量效率最高的有氧碳固定途径。Nitrosopumilus maritimus 的 4-hydroxybutyryl-CoA 合成酶(ADP-forming;Nmar_0206)是这一循环中的几种酶之一,其效率要高于子囊菌。这种酶降低了细胞对能量的需求,反映了厚朴藻在适应低营养环境方面的成功。在这里,我们展示了来自海洋硝化藻 SCM1 的 Nmar_0206 的结构,它揭示了 CoA 结合域和 ATP 抓住域之间高度保守的域间连接环。系统发生学分析表明,该环路广泛存在,并强调了它在 PDB 中的代表性不足以及在(ATP 形成的)酰基-CoA 合成酶(ACD)超家族中的结构重要性。研究表明,该连接环可能会影响结构域之间的保守界面相互作用,从而影响同源二聚体的稳定性。这些结果为这种关键酶在陶氏古菌改良的 3HP/4HB 循环中的能量效率提供了结构基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Crystal structure of the 4-hydroxybutyryl-CoA synthetase (ADP-forming) from nitrosopumilus maritimus
The 3-hydroxypropionate/4-hydroxybutyrate (3HP/4HB) cycle from ammonia-oxidizing Thaumarchaeota is currently considered the most energy-efficient aerobic carbon fixation pathway. The Nitrosopumilus maritimus 4-hydroxybutyryl-CoA synthetase (ADP-forming; Nmar_0206) represents one of several enzymes from this cycle that exhibit increased efficiency over crenarchaeal counterparts. This enzyme reduces energy requirements on the cell, reflecting thaumarchaeal success in adapting to low-nutrient environments. Here we show the structure of Nmar_0206 from Nitrosopumilus maritimus SCM1, which reveals a highly conserved interdomain linker loop between the CoA-binding and ATP-grasp domains. Phylogenetic analysis suggests the widespread prevalence of this loop and highlights both its underrepresentation within the PDB and structural importance within the (ATP-forming) acyl-CoA synthetase (ACD) superfamily. This linker is shown to have a possible influence on conserved interface interactions between domains, thereby influencing homodimer stability. These results provide a structural basis for the energy efficiency of this key enzyme in the modified 3HP/4HB cycle of Thaumarchaeota. Structural analysis suggests the importance of linkers in stability of oligomers within the (ADP-forming) Acyl-CoA Synthetase superfamily.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


