作为有效给药系统的匹铂(II)负载壳聚糖纳米复合材料:制备、BSA/5-GMP/GSH 结合的机理研究和生物学评价。
IF 2.4
3区 化学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
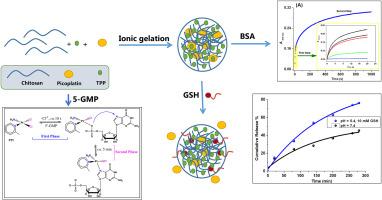
本研究的目的是通过将药物皮铂封装在壳聚糖纳米颗粒(CS NPs)中来改善皮铂的特性和生物利用度,从而将细胞毒性药物靶向输送到癌症组织中,减少毒副作用并提高治疗指数。当皮铂被递送到 CS 中时,它能够与 CS 产生一种复合物(PPt@CS NPs),该复合物的粒径为 275 ± 10 nm,PDI 值为 0.15 ± 0.05,具有较低的合理性和较高的稳定性(ζ = -22.1 ± 0.3 mV)。由于几乎所有药物都是通过与特定蛋白质或 DNA 结合而起作用的,因此利用停流和其他光谱方法研究了牛血清白蛋白(BSA)、核酸的低分子结构单位(5-GMP)和谷胱甘肽(GSH)与 PPt 和 PPt@CS NPs 的体外结合机制和亲和力(考虑到顺铂的抗药性可能是由于顺铂和 GSH 之间的反应所致)。通过两个可逆过程--快速二阶结合后的缓慢一阶异构化反应和静态淬灭机制,PPt 和 PPt@CS NPs 与 BSA 结合的相对反应活性约为(PPt)/(PPt@CS NPs)=1/2.5。5-GMP 相互作用研究表明,除了改变结合机制外,PPt 被包裹在 CS 中还能通过配位亲和力提高其反应速率。PPt 与 5-GMP 的相互作用有两个可逆过程,一个是快速的二阶结合到磷酸盐上,另一个是缓慢的一阶迁移到嘧啶分子的 N7 上。与 PPt 相比,PPt@CS NPs 与 GSH 的结合力较弱,因此 PPt@CS NPs 的抗性系数较低。研究还发现,在 pH 值为 7.4 的 PBS 溶液中,PPt@CS NPs 的体外药物释放很稳定,仅在 5 小时内就释放了 30% 的 PPt。然而,在含有 10 mM GSH 的 pH 值为 5.4 的溶液--一种模拟肿瘤微环境的溶液--中,PPt@CS NPs 释放了 75%,这表明 PPt@CS NPs 系统对 GSH 很敏感,并能特异性地靶向恶性组织。通过对 HepG2 癌细胞株进行体外细胞存活试验,发现将 PPt 包封在 CS 复合物中仍能保持其抗癌活性,而且还能通过水解作用有效地裂解 pBR322 DNA 的小沟。这些研究结果共同表明,在 CS 中加入 PPt 将是配制新型皮铂制剂的有效策略,这种制剂可用于靶向抗癌治疗。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Picoplatin (II)-loaded chitosan nanocomposites as effective drug delivery systems: Preparation, mechanistic investigation of BSA/5-GMP/GSH binding and biological evaluations
The goal of the current study is to improve the characteristics and bioavailability of the drug picoplatin (PPt) by encapsulating it in chitosan nanoparticles (CS NPs) which allows for the targeted delivery of cytotoxic cargo to cancerous tissue, reducing toxic side effects and raising the therapeutic index. When picoplatin was delivered into the CS, it was able to produce a complex with CS (PPt@CS NPs) that had an appropriate particle size of 275 ± 10 nm, a reasonably low PDI of 0.15 ± 0.05, and high stability (ζ = −22.1 ± 0.3 mV). Since almost all pharmaceuticals work by binding to specific proteins or DNA, the in vitro binding mechanism and affinity of bovine serum albumin (BSA), low molecular building units of nucleic acids (5−GMP), and Glutathione (GSH) (considering that cisplatin resistance could be due to a reaction between cisplatin and GSH) to PPt and PPt@CS NPs were examined using stopped-flow and other spectroscopic approaches. Through two reversible processes, a rapid second-order binding followed by a slower first-order isomerization reaction, and a static quenching mechanism, PPt and PPt@CS NPs bind to BSA with relative reactivity of around (PPt)/(PPt@CS NPs) = 1/2.5. The 5−GMP interaction studies demonstrated that, in addition to changing the binding mechanism, PPt's encapsulation in CS increases its rate of reaction through coordination affinity. PPt interacted with 5-GMP via two reversible processes, a rapid second-order binding to phosphate followed by a slower first−order migration to the N7 of pyrimidine moiety. PPt@CS NPs showed weaker binding to GSH compared to PPt and hence PPt@CS NPs exhibits a lower resistance factor. It was also found that the in vitro drug release of PPt@CS NPs in PBS at pH 7.4 was steady, releasing 30 % of the PPt in just 5 h. Nonetheless, 75 % of the release in a pH 5.4 solution containing 10 mM GSH—a solution that mimics the tumor microenvironment—shows that the PPt@CS NPs system is sensitive to GSH and specifically targets malignant tissue. The encapsulation of PPt in CS complex maintained its anticancer activity, as shown by an in vitro cell-survival assay on HepG2 cancer cell lines and also cleavage efficiency toward the minor groove of pBR322 DNA via the hydrolytic way. These findings collectively suggested that inclusion PPt in CS would be an effective strategy to formulate a novel picoplatin formulation intended for use as targeted anticancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Research
化学-生化与分子生物学
CiteScore
5.00
自引率
3.20%
发文量
183
审稿时长
3.6 weeks
期刊介绍:
Carbohydrate Research publishes reports of original research in the following areas of carbohydrate science: action of enzymes, analytical chemistry, biochemistry (biosynthesis, degradation, structural and functional biochemistry, conformation, molecular recognition, enzyme mechanisms, carbohydrate-processing enzymes, including glycosidases and glycosyltransferases), chemical synthesis, isolation of natural products, physicochemical studies, reactions and their mechanisms, the study of structures and stereochemistry, and technological aspects.
Papers on polysaccharides should have a "molecular" component; that is a paper on new or modified polysaccharides should include structural information and characterization in addition to the usual studies of rheological properties and the like. A paper on a new, naturally occurring polysaccharide should include structural information, defining monosaccharide components and linkage sequence.
Papers devoted wholly or partly to X-ray crystallographic studies, or to computational aspects (molecular mechanics or molecular orbital calculations, simulations via molecular dynamics), will be considered if they meet certain criteria. For computational papers the requirements are that the methods used be specified in sufficient detail to permit replication of the results, and that the conclusions be shown to have relevance to experimental observations - the authors'' own data or data from the literature. Specific directions for the presentation of X-ray data are given below under Results and "discussion".
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


