在实验性自身免疫性脑脊髓炎中,黄芪多糖通过抑制Sema4D-PlexinB2信号传导来调节小胶质细胞-星形胶质细胞之间的串扰。
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
多发性硬化症中发炎的小胶质细胞(MIMS)和多发性硬化症中发炎的星形胶质细胞(AIMS)之间的相互影响是形成中枢炎症微环境和神经毒性的关键因素。黄芪多糖(APS)是从黄芪的干燥根中提取的一种重要生物活性成分,我们的研究小组曾发现它能减轻实验性自身免疫性脑脊髓炎(EAE)小鼠(一种典型的多发性硬化症模型)中促炎性小胶质细胞的形成和神经功能障碍。为了研究APS对MIMS-AIMS串扰的影响及其内在机制,本研究建立了EAE小鼠模型和体外小胶质细胞-星形胶质细胞共培养模型。研究发现,APS能缓解EAE小鼠的神经功能障碍,并能有效抑制体内和体外MIMS和AIMS的形成。此外,研究还发现APS能抑制EAE小鼠体内MIMS-AIMS串联的炎症因子以及由此导致的体内和体外神经毒性。Sema4D-PlexinB2信号传导对于MIMS-AIMS串扰和促进中枢神经系统炎症至关重要。我们证实,APS 可抑制体内和体外的这种信号传导。在体外培养的星形胶质细胞上处理重组 Sema4D 蛋白会显著增加促炎因子和神经毒性因子,而 APS 则能显著抑制它们。相反,在敲除小胶质细胞中的 Sema4D 表达后,APS 不再能改善 MIMS-AIMS 相互交织产生的神经毒性。总之,这些结果表明,APS 可通过 Sema4D-PlexinB2 信号转导调节 MIMS-AIMS 串扰。这项研究为 APS 成为治疗脱髓鞘疾病的潜在候选药物提供了科学依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
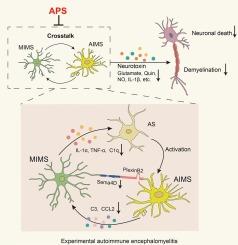
Microglia-astrocyte crosstalk is regulated by Astragalus polysaccharides mediated through suppression of Sema4D-PlexinB2 signaling in experimental autoimmune encephalomyelitis
The crosstalk between microglia inflamed in multiple sclerosis (MIMS) and astrocytes inflamed in MS (AIMS) is a crucial factor in the formation of the central inflammatory microenvironment and neurotoxicity. Astragalus polysaccharides (APS), an important bioactive component extracted from the dried root of Astragalus, was previously found by our team to attenuate the formation of pro-inflammatory microglia and neurological dysfunction in the experimental autoimmune encephalomyelitis (EAE) mice, a classic model of MS. To investigate the effect of APS on the MIMS-AIMS crosstalk and its underlying mechanism, in this study, a mouse model of EAE and a co-culture model of microglia-astrocytes in vitro were established. It was discovered that APS can alleviate the neurological dysfunction of EAE mice and effectively inhibit the formation of MIMS and AIMS both in vivo and in vitro. Furthermore, it was found that APS can suppress the inflammatory factors of MIMS-AIMS crosstalk in EAE mice and the resulting neurotoxicity in vivo and in vitro. The Sema4D-PlexinB2 signaling is essential for MIMS-AIMS crosstalk and promotes CNS inflammation. We demonstrated that APS can inhibit this signaling in vivo and in vitro. Treatment of recombinant Sema4D protein on cultured astrocytes in vitro significantly increases pro-inflammatory and neurotoxic factors, while APS significantly inhibits them. Conversely, after knockdown of Sema4D expression in microglia, APS no longer improves the neurotoxicity from MIMS-AIMS crosstalk. Overall, these results indicate that APS may modulate MIMS-AIMS crosstalk via the Sema4D-PlexinB2 signaling. This study provides a scientific basis for APS as a potential treatment candidate for demyelinating diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


