缺血性中风后糖尿病中与铁蛋白沉积相关的改变:RNA测序的启示
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
摘要
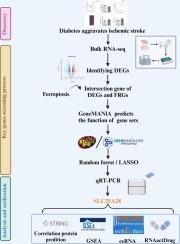
目的:铁变态反应是一种与脂质过氧化相关的铁依赖性程序性细胞死亡。虽然糖尿病会加重脑卒中的脑损伤和临床预后,但人们对铁蛋白沉积是否会导致糖尿病加重脑卒中还知之甚少。本研究旨在确定糖尿病条件下缺血性脑卒中中与铁氧化相关的差异表达基因,然后利用综合生物信息学分析探讨其作用:通过腹腔注射链脲佐菌素(110 毫克/千克),在 8-10 周龄雄性小鼠中建立 1 型糖尿病(T1D)模型。一过性 45 分钟大脑中动脉闭塞诱发缺血性中风,三天后进行评估。再灌注 24 小时后解剖缺血的大脑皮层,对其进行大量组织 RNA 测序,然后进行生物信息学分析,并通过定量实时 PCR 验证主要结果:结果:与非糖尿病小鼠相比,糖尿病小鼠的脑梗塞面积增大,同时神经系统表现恶化。与非糖尿病小鼠相比,糖尿病小鼠的体重和脾脏重量均有所下降。中风后,糖尿病小鼠的存活率呈下降趋势。在 RNA 测序分析中,我们在糖尿病小鼠和非糖尿病小鼠缺血脑中发现了 1299 个差异表达基因,分别有 732 个和 567 个基因上调和下调。在这些基因中,有 27 个基因与铁蛋白沉积有关。进一步分析发现,溶质运载家族25成员28(SLC25A28)和固醇运载蛋白2(SCP2)是缺血性脑卒中后糖尿病小鼠中与铁蛋白沉积相关的最大基因。在几项生物信息学分析中,我们发现 SLC25A28 作为与铁突变相关的顶级基因之一,参与了多个代谢和调控通路,以及微RNA 和环状 RNA 的复杂调控,这表明 SLC25A28 在糖尿病加重脑卒中中的潜在作用。药物网络分析表明,SLC25A28是改善糖尿病缺血性损伤的潜在治疗靶点:结论:我们的大容量 RNA 测序和生物信息学分析表明,铁突变信号通路的改变与糖尿病条件下实验性脑卒中损伤的加重有关。结论:我们的大容量 RNA 测序和生物信息学分析表明,铁氧化酶信号通路的改变与糖尿病条件下实验性脑卒中损伤的加重有关,尤其是 SLC25A28 和 SCP2 在糖尿病加重脑卒中中的作用机制有待进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Ferroptosis-associated alterations in diabetes following ischemic stroke: Insights from RNA sequencing
Objective
Ferroptosis is an iron-dependent form of programmed cell death associated with lipid peroxidation. Though diabetes worsens cerebral injury and clinical outcomes in stroke, it is poorly understood whether ferroptosis contributes to diabetes-exacerbated stroke. This study aimed to identify ferroptosis-associated differentially expressed genes in ischemic stroke under diabetic condition and then explore their roles using comprehensive bioinformatics analyses.
Methods
Type 1 diabetes (T1D) model was established in male mice at 8–10 weeks of age by one intraperitoneal injection of streptozotocin (110 mg/kg). Ischemic stroke was induced by a transient 45-minute middle cerebral artery occlusion and evaluated three days thereafter. Ischemic brain cortex was dissected 24 h after the reperfusion and subjected to bulk tissue RNA sequencing followed by bioinformatics analysis and verification of key findings via quantitative real-time PCR.
Results
Enlarged infarct size was seen in diabetic, as compared with non-diabetic mice, in conjunction with worsened neurological behaviors. Both body and spleen weights were reduced in diabetic as compared with non-diabetic mice. There was a trend for reduced survival rate in diabetic mice following the stroke. In RNA sequencing analysis, we identified 1299 differentially expressed genes in ischemic brain between diabetic and non-diabetic mice, with upregulation and downregulation for 732 and 567 genes, respectively. Among these genes, 27 genes were associated with ferroptosis. Further analysis reveals that solute carrier family 25 member 28(SLC25A28) and sterol carrier protein 2(SCP2) were the top genes associated with ferroptosis in diabetic mice following ischemic stroke. In several bioinformatics analyses, we found SLC25A28, one of the top ferroptosis-related genes, is involved in several metabolic and regulatory pathways as well as the regulatory complexity of microRNAs and circular RNAs, which demonstrates the potential role of SLC25A28 in diabetes-exacerbated stroke. Drug network analysis suggests SLC25A28 as a potential therapeutic target for ameliorating ischemic injury in diabetes.
Conclusions
Our bulk RNA sequencing and bioinformatics analyses show that altered ferroptosis signaling pathway was associated with the exacerbation of experimental stroke injury under diabetic condition. Especially, additional investigation into the mechanisms of SLC25A28 and SCP2 in diabetes-exacerbated stroke will be explored in the future study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


