阳离子两亲分子作为治疗静脉畸形的新型硬化剂:氨甲环酸衍生低分子量凝胶研究。
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-15
DOI:10.1016/j.bbrc.2024.150838
引用次数: 0
摘要
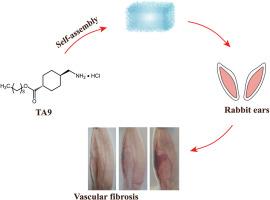
静脉畸形(VM)是一种常见的先天性血管畸形,其特点是血管异常增生,导致毁容和功能障碍。硬化剂注射疗法是一种微创疗法,已成为静脉畸形的主要治疗方法,但其疗效受到快速稀释和潜在不良反应的影响。在这项研究中,我们引入了一系列阳离子两亲分子--氨甲环酸(TA)的脂肪醇酯(TA6、TA8 和 TA9),它们能在水中自组装成低分子量凝胶(LMWGs)。尤其是 TA9,当水凝胶被注入静脉局部时会缓慢释放。然后,它通过破坏细胞膜和沉淀蛋白质来破坏静脉壁,引起炎症和血栓形成、静脉壁增厚,有效地诱发不可逆的静脉纤维化。此外,TA9 可在血浆中迅速降解为 TA,以减少扩散引起的毒性。总之,这项研究表明,阳离子两亲分子 TA9 是一种很有前景的治疗血管瘤的硬化剂,提供了一种新颖、有效、安全的治疗选择,具有临床转化的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Cationic amphiphilic molecules as novel sclerosants for venous malformation treatment: A study on tranexamic acid-derived low-molecular-weight gels
Venous malformation (VM) is a prevalent congenital vascular anomaly characterized by abnormal blood vessel growth, leading to disfigurement and dysfunction. Sclerotherapy, a minimally invasive approach, has become a primary therapeutic modality for VM, but its efficacy is hampered by the rapid dilution and potential adverse effects. In this study, we introduced a series of cationic amphiphilic molecules, fatty alcohol esters (TA6, TA8, and TA9) of tranexamic acid (TA), which self-assembled into low-molecular-weight gels (LMWGs) in water. The TA9, in particular, is released slowly when hydrogel is injected into the vein locally. Then, it damages the venous wall by destroying cell membranes and precipitating proteins, causing inflammation and thrombosis, thickening of the venous wall, effectively inducing irreversible vein fibrosis. Additionally, TA9 can be rapidly degraded into TA in plasma to reduce toxicity caused by diffusion. Overall, this study suggests that the cationic amphiphilic molecule TA9 is a promising sclerosant for VM treatment, offering a novel, effective, and safe therapeutic option with potential for clinical translation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


