比特拉韦会改变体内葡萄糖耐量并导致肝线粒体功能障碍。
IF 4.5
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
越来越多的证据表明,含有整合酶链转移抑制剂或替诺福韦-阿拉非那胺(TAF)的抗逆转录病毒疗法与体重增加和代谢性疾病有关,但其潜在机制仍不清楚。本研究评估了拉米夫定、多替拉韦(DTG)、比特拉韦(BIC)、富马酸替诺福韦二吡呋酯和 TAF 对代谢改变的影响,并探讨了作为潜在机制的葡萄糖稳态和线粒体应激。对这些途径进行了体内(C57BL/6J小鼠,用上述药物或载体治疗16周)和体外(Hep3B细胞)分析。在葡萄糖耐量试验中,接受 BIC 治疗的小鼠表现出更高的葡萄糖水平和更慢的葡萄糖下降速度。对抗逆转录病毒治疗小鼠肝脏的功能富集分析表明,只有 BIC 改变了细胞对胰岛素的反应,并诱导了有利于葡萄糖生成的特征,其中 Fgf21 起了重要作用。在体外,无论是在基础条件下还是在胰岛素刺激后,BIC 都能以浓度依赖的方式显著降低肝细胞的葡萄糖摄取,而其他药物则不会产生显著变化。用临床相关浓度的 BIC 处理 Hep3B 细胞后,与葡萄糖代谢有关的酶的 mRNA 表达发生了明显变化。DTG 和 BIC 都降低了线粒体脱氢酶的活性,但只有 BIC 增加了活性氧、线粒体膜电位和细胞颗粒度,从而显示线粒体应激。BIC 促进了线粒体功能障碍,改变了肝细胞的碳水化合物代谢和葡萄糖消耗,并改变了小鼠的糖耐量和糖生成调节。这些研究结果表明,BIC 会导致艾滋病毒感染者的胰岛素抵抗和糖尿病,因此有必要进行临床研究,以明确其与碳水化合物代谢紊乱的关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
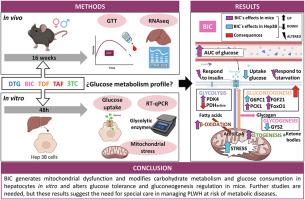
Bictegravir alters glucose tolerance in vivo and causes hepatic mitochondrial dysfunction
Growing evidence associates antiretroviral therapies containing integrase strand transfer inhibitors or tenofovir alafenamide (TAF) with increased weight gain and metabolic diseases, but the underlying mechanisms remain unclear. This study evaluated the impact of lamivudine, dolutegravir (DTG), bictegravir (BIC), tenofovir disoproxil fumarate, and TAF on metabolic alterations, and explored glucose homeostasis and mitochondrial stress as potential mechanisms. These pathways were analyzed both in vivo (C57BL/6J mice treated with the abovementioned drugs or vehicle for 16 weeks) and in vitro (in Hep3B cells). Mice treated with BIC exhibited higher glucose levels and a slower decrease during a glucose tolerance test. Functional enrichment analyses of livers from antiretroviral-treated mice revealed that only BIC altered the cellular response to insulin and induced a gluconeogenic-favoring profile, with Fgf21 playing a significant role. In vitro, BIC significantly reduced hepatocyte glucose uptake in a concentration-dependent manner, both under basal conditions and post-insulin stimulation, while the other drugs produced no significant changes. Hep3B cells treated with clinically relevant concentrations of BIC exhibited significant alterations in the mRNA expression of enzymes related to glucose metabolism. Both DTG and BIC reduced mitochondrial dehydrogenase activity, but only BIC increased reactive oxygen species, mitochondrial membrane potential, and cellular granularity, thereby indicating mitochondrial stress. BIC promoted mitochondrial dysfunction, modified carbohydrate metabolism and glucose consumption in hepatocytes, and altered glucose tolerance and gluconeogenesis regulation in mice. These findings suggest that BIC contributes to insulin resistance and diabetes in people living with HIV, warranting clinical studies to clarify its association with carbohydrate metabolism disorders.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Antiviral research
医学-病毒学
CiteScore
17.10
自引率
3.90%
发文量
157
审稿时长
34 days
期刊介绍:
Antiviral Research is a journal that focuses on various aspects of controlling viral infections in both humans and animals. It is a platform for publishing research reports, short communications, review articles, and commentaries. The journal covers a wide range of topics including antiviral drugs, antibodies, and host-response modifiers. These topics encompass their synthesis, in vitro and in vivo testing, as well as mechanisms of action. Additionally, the journal also publishes studies on the development of new or improved vaccines against viral infections in humans. It delves into assessing the safety of drugs and vaccines, tracking the evolution of drug or vaccine-resistant viruses, and developing effective countermeasures. Another area of interest includes the identification and validation of new drug targets. The journal further explores laboratory animal models of viral diseases, investigates the pathogenesis of viral diseases, and examines the mechanisms by which viruses avoid host immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


