生物素化人源化抗柯卡因 mAb 的溶解度降低,吸附性增强。
IF 2.6
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
蛋白质(包括抗体)的生物素化是一种非常有用和重要的修饰,可用于各种生化鉴定,包括用于检测针对治疗性抗体的抗药物抗体(ADA)检测。我们评估了目前正在开发用于治疗可卡因使用障碍的抗可卡因 mAb 的不同生物素标记程度。我们注意到,生物素标记程度越高,mAb 的溶解度就越低,与表面结合的倾向就越大,从而使生物素化抗体的表征变得更加复杂。具体来说,在磷酸盐缓冲盐水中,生物素/mAb 的标记比例超过 3 时,由于与表面的结合增加和 mAb 溶解度降低,回收率会下降。原生凝胶琼脂糖电泳、差示扫描荧光测定法和等温滴定量热法都显示了 mAb 的变化,当生物素/mAb 的标记比率超过 3 时,这种变化会更加明显。在生物素/mAb 为 3.0 时,溶解度、吸附性、疏水性染料结合位点的暴露、热稳定性和可卡因结合力的变化都很小,这与生物素/mAb 为 5.6 时的标记形成了鲜明对比。因此,生物素化程度应保持在 3 生物素/mAb 左右,以维持这种 mAb 的抗原结合力、一般结构、溶解性和稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
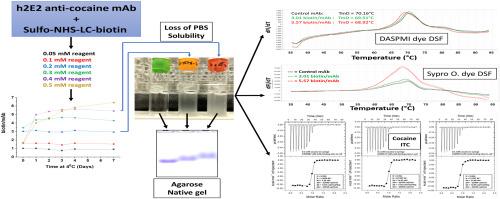
Decreased solubility and increased adsorptivity of a biotinylated humanized anti-cocaine mAb
Biotinylation of proteins, including antibodies, is a very useful and important modification for a variety of biochemical characterizations, including anti-drug antibody (ADA) assays used to detect antibodies raised against therapeutic antibodies. We assessed different degrees of biotin labeling of an anti-cocaine mAb currently under development for treating cocaine use disorder. We noted that higher levels of biotin labeling dramatically decreased mAb solubility, and increased the tendency to bind to surfaces, complicating characterization of the biotinylated antibody. Specifically, in phosphate buffered saline, labeling stoichiometries of more than about 3 biotin/mAb resulted in decreased recoveries due to increased binding to surfaces and decreased mAb solubility. Native gel agarose electrophoresis, differential scanning fluorimetry, and isothermal titration calorimetry all demonstrated changes in the mAb which became more pronounced above a labeling ratio of 3 biotin/mAb. At 3.0 biotin/mAb, there were minimal changes in solubility, adsorptivity, exposure of hydrophobic dye-binding sites, heat stability, and cocaine binding, in stark contrast to labeling with 5.6 biotin/mAb. Thus, the degree of biotinylation should be kept at about 3 biotin/mAb to maintain antigen binding and general structure, solubility, and stability of this mAb, a finding which may be important for other similar mAbs currently in use or under development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


