细胞钙化可通过糖脂代谢转换逆转癌细胞的化疗抗性。
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
抗药性是癌症化疗过程中固有的挑战。癌细胞通过新陈代谢重编程来促进脂肪酸代谢,从而实现抗药性。然而,克服从依赖糖酵解到依赖脂肪酸β-氧化的合成代谢和能量代谢转换的有效方法仍未出现。在此,我们开发了一种大分子药物(叶酸-聚矽氧烷,FpSA)来诱导顺铂耐药的宫颈癌细胞细胞外微钙化。微钙化削弱了线粒体功能障碍对脂肪酸的吸收和脂肪酸的β-氧化,但促进了糖酵解途径。因此,铂和FpSA的协同处理抑制了顺铂耐药肿瘤的生长,提高了肿瘤小鼠的存活率,这表明FpSA将脂肪酸代谢转换为糖酵解,使顺铂耐药细胞进一步敏化。综上所述,FpSA诱导的癌细胞钙化为治疗化疗耐药肿瘤提供了一种重编程代谢策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
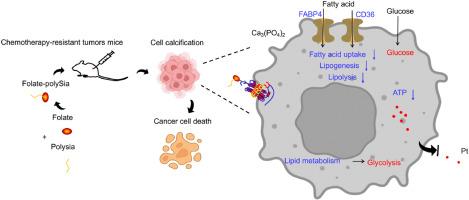
Cell calcification reverses the chemoresistance of cancer cells via the conversion of glycolipid metabolism
Drug resistance is an inherent challenge during cancer chemotherapy. Cancer cells favor fatty acid metabolism through metabolic reprogramming to achieve therapeutic resistance. However, an effective approach to overcoming the switch from glycolysis-dependent to fatty acid beta-oxidation-dependent anabolic and energy metabolism remains elusive. Here, we developed a macromolecular drug (folate-polySia, FpSA) to induce the extracellular microcalcification of cervical cancer cells with cisplatin resistance. Microcalcification attenuated the uptake of fatty acids and the beta-oxidation of fatty acids by mitochondrial dysfunction but boosted the glycolysis pathway. Consequently, cotreatment with Pt and FpSA inhibited cisplatin-resistant tumor growth and improved tumor-bearing mice's survival rates, indicating that FpSA switched fatty acid metabolism to glycolysis to sensitize cisplatin-resistant cells further. Taken together, cancer cell calcification induced by FpSA provides a reprogramming metabolic strategy for the treatment of chemotherapy-resistant tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


