通过 C-H 功能化合成双芳基咔唑并探索其抗癌活性。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究报道了通过双叉定向基团辅助 C-H 功能化合成新的双芳基咔唑库,并对双芳基咔唑的抗癌特性进行了初步筛选。虽然各类改性咔唑因其在材料和药物化学中的应用而广为人知,但据我们所知,设计的双芳基咔唑的生物活性却鲜为人知。鉴于咔唑在药物化学研究中的突出地位,我们设想了基于咔唑的双芳基结构新支架的应用范围。我们筛选了合成的基于双芳基的咔唑对各种癌细胞系的抗癌特性,如 HeLa(宫颈癌)、HCT116(结肠癌)、MDA-MB-231 和 MDA-MB-468(乳腺癌)。此外,我们还在人类胚胎肾细胞系 HEK293T 中测试了这些化合物,以评估它们在这些化合物存在的情况下对正常人体细胞活力的影响。在这项初步研究中,我们发现一些基于双芳基的咔唑类化合物具有抗癌活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
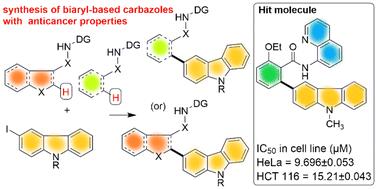
Synthesis of biaryl-based carbazoles via C–H functionalization and exploration of their anticancer activities†
The synthesis of a library of new biaryl-based carbazoles via bidentate directing group-assisted C–H functionalization and preliminary screening of the anticancer properties of biaryl-based carbazoles is reported. While various classes of modified carbazoles are known for their applications in materials and medicinal chemistry, to our knowledge, the biological activities of designed biaryl-based carbazoles have been rarely known. Given the prominence of carbazoles in research in medicinal chemistry, we envisioned the scope for new scaffolds of carbazole-based biaryl structures. We screened the synthesized biaryl-based carbazoles for their anticancer properties against various cancer cell lines such as HeLa (cervical cancer), HCT116 (colon cancer), MDA-MB-231 and MDA-MB-468 (breast cancer). In addition, the hits were also tested in the human embryonic kidney cell line HEK293T to assess their impact on the viability of normal human cells in the presence of these compounds. In this preliminary study, we identified some of the biaryl-based carbazoles as lead compounds with anticancer activities.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


