MeOTf 催化的苯并恶唑酮、苯并噻唑酮、吲哚啉酮和苯并咪唑酮与活化仲醇的 Friedel-Crafts 烷基化反应。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
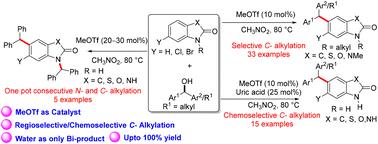
在此,我们揭示了一种在三氟甲磺酸甲酯(MeOTf)催化下结合活化醇对苯并恶唑酮、苯并噻唑酮、吲哚啉酮和苯并咪唑酮进行选择性 C- 烷基化的方法。这种方法为合成烷基化杂环提供了一种绿色、原子经济的替代方法,唯一的副产品是水。醇类因其丰富、易于制备和环保,已成为极具吸引力的烷基化剂。所开发的反应条件不仅产率高,而且广泛适用于各种 N-烷基化杂环,凸显了 MeOTf 催化的多功能性。该方法还适用于含有游离 -NH 基团的杂环的单锅连续 N- 烷基化和 C- 烷基化以及化学选择性 C- 烷基化。这种方法为生物活性杂环化合物的官能化提供了一条实用而高效的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
MeOTf-catalyzed Friedel–Crafts alkylation of benzoxazolones, benzothiazolones, indolinones and benzimidazolones with activated secondary alcohols†
Herein, we have revealed a methodology for the selective C-alkylation of benzoxazolones, benzothiazolones, indolinones, and benzimidazolones incorporating activated alcohols catalysed by methyltrifluoromethanesulfonate (MeOTf). This method offers a green, atom-economic alternative for the synthesis of alkylated heterocycles, producing water as the only byproduct. Alcohols, due to their abundance, ease of preparation, and environmental friendliness, have become attractive alkylating agents. The developed reaction conditions demonstrate high yields and broad applicability across various N-alkylated heterocycles, highlighting the versatility of the MeOTf catalysis. The method was also adapted for one-pot consecutive N- and C-alkylation and chemoselective C-alkylation in heterocycles containing a free –NH group. This approach provides a practical and efficient route for the functionalization of bioactive heterocyclic compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


