丹参酮 IIA 的电化学氧化位点选择性直接 C-H 活化。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
天然产品在最先进药物的研发过程中发挥着举足轻重的作用。为了增强疗效,人们经常对这些化合物进行结构改造。在本研究中,我们介绍了一种高效且对环境无害的电化学氧化方法,用于在无金属、无氧化剂和无碱条件下对丹参酮 IIA 进行位点选择性直接 C-H 活化。在广泛的底物范围内,获得了所需的丹参酮 IIA 衍生物,产率从中等到极佳,最高可达 92%。生物活性实验证明,与丹参酮 IIA 相比,化合物 2k、2q 和 2w 具有更强的抗肿瘤功效。本文章由计算机程序翻译,如有差异,请以英文原文为准。
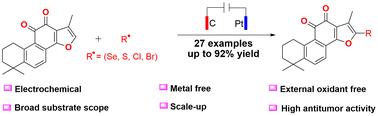
Electrochemical oxidative site-selective direct C–H activation of tanshinone IIA†
Natural products play a pivotal role in the advancement of state-of-the-art pharmaceuticals. To augment their therapeutic efficacy, structural modifications of these compounds are routinely performed. In this study, we introduce an efficient and environmentally benign electrochemical oxidative method for site-selective direct C–H activation of tanshinone IIA under metal-free, oxidant-free, and base-free conditions. Moderate to excellent yields up to 92% of the desired tanshinone IIA derivatives were obtained with a broad substrate scope. Biological activity assays demonstrate that compounds , and possess superior antitumor efficacy compared to tanshinone IIA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


