通过光催化与烷烃偶联实现三氟甲基烯的化学歧化烷基化。
IF 9.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
gem-二氟烯烃和三氟甲基烷烃是生物活性化合物中的重要结构。α-三氟甲基烯的自由基烷基化是获得这些结构的有效策略。然而,已报道的方法都依赖于使用预官能化的自由基前体,而使用简单碳氢化合物作为偶联剂的例子却很难找到。在此,我们报告了一种基于 HAT 光氧化催化直接活化 C(sp3)-H 键的化学变异方法。该方法为从包括气态烷烃在内的各种烃类原料中制备二氟烯烃和三氟甲基烷烃提供了一个高效平台。重要的是,只需简单改变反应条件和/或添加剂,就能轻松实现化学选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
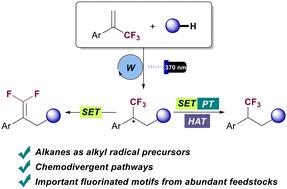
Chemodivergent alkylation of trifluoromethyl alkenes via photocatalytic coupling with alkanes†
gem-Difluoroalkenes and trifluoromethyl alkanes are prominent structures in biologically active compounds. Radical alkylation of α-trifluoromethyl alkenes represents a useful strategy to access these structures. However, reported methods have relied on the use of pre-functionalized radical precursors and examples involving the use of simple hydrocarbons as coupling partners are elusive. Here we report a chemodivergent methodology based on the direct activation of C(sp3)–H bonds enabled by HAT photoredox catalysis. This protocol provides an efficient platform for preparing both gem-difluoroalkenes and trifluoromethyl alkanes from ubiquitous hydrocarbon feedstocks, including gaseous alkanes. Importantly, chemoselectivity is easily achieved by simple modification of reaction conditions and/or additives.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


