Vax-Innate:通过调节 T 细胞和肿瘤微环境改进治疗性癌症疫苗
IF 67.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
T 细胞在介导抗肿瘤免疫方面发挥着关键作用。免疫检查点抑制剂(ICIs)在治疗癌症方面取得的成功突显了增强内源性 T 细胞反应如何介导肿瘤消退。然而,许多癌症的死亡率仍然很高,尤其是转移性癌症。根据肿瘤基因特征描述和肿瘤特异性抗原鉴定方面的进展,目前正在开发针对变异肿瘤抗原(新抗原)的个体化治疗性癌症疫苗,以产生肿瘤特异性 T 细胞,改善治疗反应。针对晚期疾病患者使用个体化新抗原疫苗的早期临床试验尽管已证明能诱导 T 细胞应答,但临床疗效有限。因此,通过提高疫苗接种后 T 细胞应答的幅度、质量和广度来增强 T 细胞活性,是目前改善转移性肿瘤治疗效果的目标之一。另一个主要考虑因素是如何进一步优化 T 细胞在肿瘤微环境 (TME) 中的功能。在本《视角》中,我们将重点放在新抗原疫苗上,并提出了一种称为 Vax-Innate 的新方法,即通过静脉注射疫苗或与肿瘤靶向免疫调节剂联合接种疫苗,可同时提高 T 细胞的数量、质量和广度,并将 TME 转变为主要针对 T 细胞的免疫刺激环境,从而提高抗肿瘤疗效。本文章由计算机程序翻译,如有差异,请以英文原文为准。

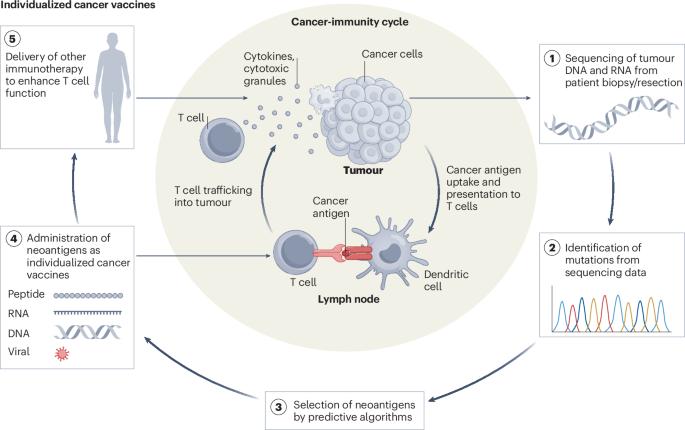
Vax-Innate: improving therapeutic cancer vaccines by modulating T cells and the tumour microenvironment
T cells have a critical role in mediating antitumour immunity. The success of immune checkpoint inhibitors (ICIs) for cancer treatment highlights how enhancing endogenous T cell responses can mediate tumour regression. However, mortality remains high for many cancers, especially in the metastatic setting. Based on advances in the genetic characterization of tumours and identification of tumour-specific antigens, individualized therapeutic cancer vaccines targeting mutated tumour antigens (neoantigens) are being developed to generate tumour-specific T cells for improved therapeutic responses. Early clinical trials using individualized neoantigen vaccines for patients with advanced disease had limited clinical efficacy despite demonstrated induction of T cell responses. Therefore, enhancing T cell activity by improving the magnitude, quality and breadth of T cell responses following vaccination is one current goal for improving outcome against metastatic tumours. Another major consideration is how T cells can be further optimized to function within the tumour microenvironment (TME). In this Perspective, we focus on neoantigen vaccines and propose a new approach, termed Vax-Innate, in which vaccination through intravenous delivery or in combination with tumour-targeting immune modulators may improve antitumour efficacy by simultaneously increasing the magnitude, quality and breadth of T cells while transforming the TME into a largely immunostimulatory environment for T cells. This Perspective considers present and historical paradigms of therapeutic cancer vaccines and describes a conceptual framework, termed Vax-Innate, to simultaneously generate robust tumour-specific T cell responses and remodel the suppressive tumour microenvironment (TME). The authors detail how this strategy could be achieved through systemic vaccination and by using immune modulators to improve dendritic cell and macrophage function in the TME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Immunology
医学-免疫学
CiteScore
93.40
自引率
0.40%
发文量
131
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Immunology is a journal that provides comprehensive coverage of all areas of immunology, including fundamental mechanisms and applied aspects. It has two international standard serial numbers (ISSN): 1474-1733 for print and 1474-1741 for online. In addition to review articles, the journal also features recent developments and new primary papers in the field, as well as reflections on influential people, papers, and events in the development of immunology. The subjects covered by Nature Reviews Immunology include allergy and asthma, autoimmunity, antigen processing and presentation, apoptosis and cell death, chemokines and chemokine receptors, cytokines and cytokine receptors, development and function of cells of the immune system, haematopoiesis, infection and immunity, immunotherapy, innate immunity, mucosal immunology and the microbiota, regulation of the immune response, signalling in the immune system, transplantation, tumour immunology and immunotherapy, and vaccine development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


