营养过剩会增加交感神经系统的活动,导致胰岛素抵抗和代谢紊乱
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
肥胖诱发胰岛素抵抗的机制仍不完全清楚,因为细胞胰岛素信号传递受损(传统上被认为是胰岛素抵抗的主要驱动因素)并不总是伴随着胰岛素作用受损。营养过剩会迅速增加血浆去甲肾上腺素(NE),这表明交感神经系统(SNS)被过度激活。然而,交感神经系统在肥胖中的作用还存在争议,因为交感神经系统活性(SNA)的增加和减少均有报道。在这里,我们利用诱导性和外周限制性酪氨酸羟化酶(th;THΔper)缺失的小鼠模型证明,减少交感神经系统释放儿茶酚胺(CA)可防止营养过剩引起的胰岛素抵抗以及高胰高血糖素血症、脂肪组织功能障碍和脂肪肝。SNA 增高诱导胰岛素抵抗的一个关键机制是引发脂肪组织脂肪分解。在营养过剩诱发的胰岛素抵抗和代谢性疾病的发病机制中,SNA 的增加是一个关键的驱动因素,与细胞胰岛素信号无关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
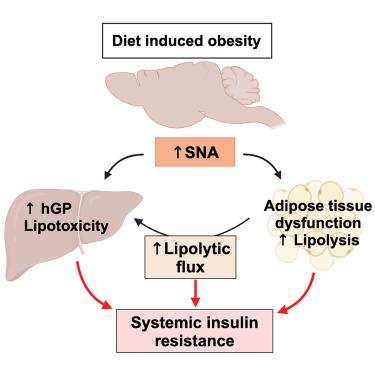
Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity
The mechanisms underlying obesity-induced insulin resistance remain incompletely understood, as impaired cellular insulin signaling, traditionally considered the primary driver of insulin resistance, does not always accompany impaired insulin action. Overnutrition rapidly increases plasma norepinephrine (NE), suggesting overactivation of the sympathetic nervous system (SNS). However, the role of the SNS in obesity is controversial, as both increased and decreased SNS activity (SNA) have been reported. Here, we show that reducing catecholamine (CA) release from the SNS protects against overnutrition-induced insulin resistance as well as hyperglucagonemia, adipose tissue dysfunction, and fatty liver disease, as we demonstrate utilizing a mouse model of inducible and peripherally restricted deletion of tyrosine hydroxylase (th; THΔper). A key mechanism through which heightened SNA induces insulin resistance is by triggering adipose tissue lipolysis. Increased SNA emerges as a critical driver in the pathogenesis of overnutrition-induced insulin resistance and metabolic disease independent of cellular insulin signaling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


