新型环状氮杂蒽的合成进展及其独特的药理特性
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
环状氮杂吖啶,主要是部分饱和的苯并[d]氮杂吖啶和二苯并[c,g]氮杂吖啶融合异构体,是一类独特的生物碱和受自然启发的氮杂环化合物,具有有趣的反应活性、物理化学和生物学特性。由于苯并吖嗪(或生物异构)支架合成的困难,它们并不是有机和药物化学家考虑的重点,但值得注意的是它们的药理活性范围及其在药物化学中的潜在应用。在此,我们回顾了迄今已知的炔融合氮杂蒽衍生物的合成方法,并报告了在揭示其药物发现潜力方面取得的进展。事实上,它们的构象限制或释放促使它们对不同的生物靶点具有选择性,使它们成为开发药物的多功能支架,包括抗精神病药物和抗癌药物,以及具有抗神经退行性治疗潜力的小分子药物,正如最近的文献所显示的那样。本文章由计算机程序翻译,如有差异,请以英文原文为准。
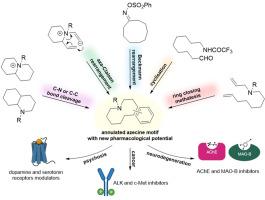
Advances in synthesis of novel annulated azecines and their unique pharmacological properties
Annulated azecines, mostly partially saturated benzo[d]azecine and dibenzo[c,g]azecine fusion isomers, constitute a unique class of alkaloids and nature-inspired azaheterocyclic compounds with interesting reactivity, physicochemical and biological properties. Due to difficulties associated with the synthesis of the benzazecine (or bioisosteric) scaffold they are not the focus of organic and medicinal chemists' consideration, whereas it is worth noting the range of their pharmacological activities and their potential application in medicinal chemistry. Herein, we reviewed the synthetic methodologies of arene-fused azecine derivatives known up to date and reported about the progress in disclosing their potential in drug discovery. Indeed, their conformational restriction or liberation drives their selectivity towards diverse biological targets, making them versatile scaffolds for developing drugs, including antipsychotic and anticancer drugs, but also small molecules with potential for anti-neurodegenerative treatments, as the recent literature shows.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


