铜催化 1,3-二烯的 1,4-氨基羟化反应和羰基辅助迁移的氨基硫代反应
IF 11.6
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
我们报告了铜催化的 1,3-二烯 1,4 选择性氨基氧化反应,它是 1,4- 烯丙基氨基醇的直接入口。该反应对各种含酰胺、脲和酯的 1,3-二烯都有效,可以方便地加入脂肪族烷基胺和游离醇。这种转化由铜催化的亲电胺化反应(使用 O-苯甲酰基羟胺)启动,在随后的氧合步骤中,羰基辅助的氧迁移提供了独有的 1,4 选择性。受这些机理见解的启发,我们还利用新颖的硫醇迁移技术,对含硫代酰胺的 1,3-二烯进行了前所未有的 1,4-氨基硫代反应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
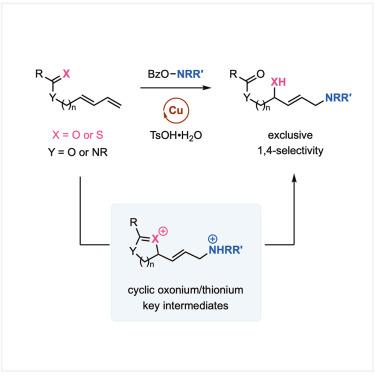
Copper-catalyzed 1,4-aminohydroxylation and aminothiolation of 1,3-dienes by carbonyl-assisted migration
We report a copper-catalyzed 1,4-selective aminooxygenation of 1,3-dienes as a direct entry to 1,4-allylic amino alcohols. The reactions are effective on a diverse range of amide-, urea-, and ester-containing 1,3-dienes, allowing for the facile installation of aliphatic alkylamines and free alcohol. The transformation was initiated by a copper-catalyzed electrophilic amination using O-benzoylhydroxylamines, and a carbonyl-assisted oxygen migration delivered the exclusive 1,4-selectivity in the subsequent oxygenation step. Inspired by these mechanistic insights, we also realized an unprecedented 1,4-aminothiolation of thioamide-containing 1,3-dienes by leveraging a novel thiol migration.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


