双酶驱动级联反应调节免疫抑制性肿瘤微环境,促进催化治疗和免疫激活
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
乳酸盐富集的肿瘤微环境(TME)会形成一种免疫抑制环境,阻碍肿瘤相关巨噬细胞(TAMs)功能的发挥。然而,如何解决乳酸积累带来的免疫抑制效应仍是一个巨大的挑战。在此,我们构建了一个具有免疫抑制TME调节功能的双酶驱动级联反应平台(ILH),用于光声(PA)成像引导的催化治疗和免疫激活。ILH由铱(Ir)金属纳米酶、乳酸氧化酶(LOx)和透明质酸(HA)组成。铱金属纳米酶和乳酸氧化酶的结合不仅能有效消耗乳酸,将免疫抑制的TME逆转为免疫活性的TME,促进TAMs从M2表型极化为M1表型,从而增强抗肿瘤防御能力,还能缓解肿瘤缺氧,诱导强氧化应激,从而引发免疫原性细胞死亡(ICD),激活抗肿瘤免疫。此外,Ir 纳米酶的光热性能可增强级联催化能力,赋予 ILH PA 响应。根据内源性分子 PA 信号的变化,利用三维多光谱 PA 成像追踪体内级联催化治疗的过程。这项研究为酶驱动级联催化疗法和通过调节免疫抑制TME激活免疫提供了一个纳米平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
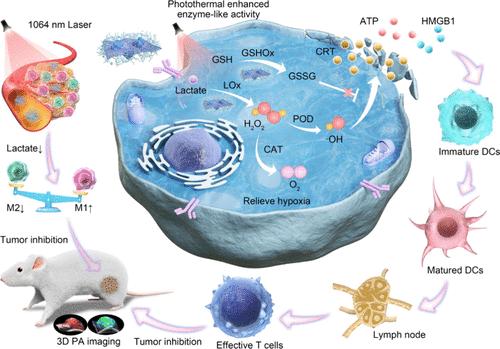
Dual Enzyme-Driven Cascade Reactions Modulate Immunosuppressive Tumor Microenvironment for Catalytic Therapy and Immune Activation
Lactate-enriched tumor microenvironment (TME) fosters an immunosuppressive milieu to hamper the functionality of tumor-associated macrophages (TAMs). However, tackling the immunosuppressive effects wrought by lactate accumulation is still a big challenge. Herein, we construct a dual enzyme-driven cascade reaction platform (ILH) with immunosuppressive TME modulation for photoacoustic (PA) imaging-guided catalytic therapy and immune activation. The ILH is composed of iridium (Ir) metallene nanozyme, lactate oxidase (LOx), and hyaluronic acid (HA). The combination of Ir nanozyme and LOx can not only efficiently consume lactate to reverse the immunosuppressive TME into an immunoreactive one by promoting the polarization of TAMs from the M2 to M1 phenotype, thus enhancing antitumor defense, but also alleviate tumor hypoxia as well as induce strong oxidative stress, thus triggering immunogenic cell death (ICD) and activating antitumor immunity. Furthermore, the photothermal performance of Ir nanozyme can strengthen the cascade catalytic ability and endow ILH with a PA response. Based on the changes in PA signals from endogenous molecules, three-dimensional multispectral PA imaging was utilized to track the process of cascade catalytic therapy in vivo. This work provides a nanoplatform for dual enzyme-driven cascade catalytic therapy and immune activation by regulating the immunosuppressive TME.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


