通过双极耦合驱动 DNA 纳米孔膜插入
IF 9.6
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
DNA 纳米孔似乎是使用跨膜蛋白的一种可行的替代方法。DNA 相互作用的特异性和可编程性使我们能够设计出插入脂质双分子层的合成通道,并能调节离子在其中的传输。在这篇通讯中,我们研究了插入能力与纳米孔静电特性的关系,结果表明永久偶极子的存在是纳米孔插入膜的重要因素。相反,在没有这种偶极的情况下,大多数 DNA 纳米孔会与双分子层结合而不会形成通道。我们还发现,这种修饰并不妨碍通过单个短寡核苷酸引发可测量的构象变化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
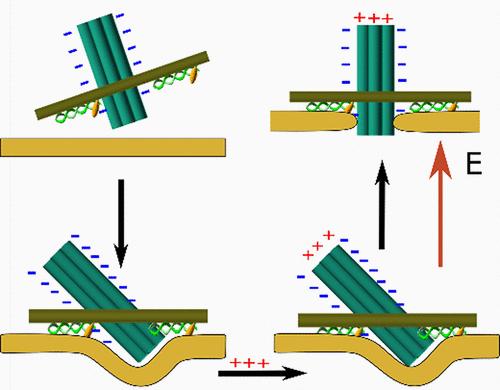
Driving DNA Nanopore Membrane Insertion through Dipolar Coupling
DNA nanopores appear to be a plausible alternative to the use of transmembrane proteins. The specificity and programmability of DNA interactions allow the design of synthetic channels that insert into lipid bilayers and can regulate the ionic transport across them. In this Communication, we investigate the dependence of insertion capabilities on the electrostatic properties of the nanopore and show that the presence of a permanent electric dipole is an important factor for the nanopore to insert into the membrane. On the contrary, in the absence of such a dipole, most DNA nanopores bind to the bilayer without channel formation. We also show that this modification does not hinder the possibility of triggering a measurable conformational change with a single short oligonucleotide.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


