基于四内酰胺的阴离子转运体
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
合成阴离子转运体为治疗囊性纤维化和癌症等疾病提供了一条前景广阔的途径。阴离子结合位点预组织是转运体设计的一个方面,可以通过操作来增强结合。大环具有预组织的结合空腔,可实现更有选择性和更高效的阴离子结合和转运。在本研究中,我们以大环四内酰胺为支架,制备了一系列含氟和不含氟的四内酰胺阴离子转运体。阴离子结合和转运试验用于分析取代基对支架亲油性、选择性、溶解性、结合强度和转运率的影响。分析了该系列化合物与 Cl- 和 F- 阴离子结合并通过脂质双分子层转运的能力。在 8-羟基芘-1,3,6-三磺酸 (HPTS) 试验和基于 Eu(III) 探针的 F- 运输试验中,一些高氟化四内酰胺显示出极高的 Cl- 和 F- 运输活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
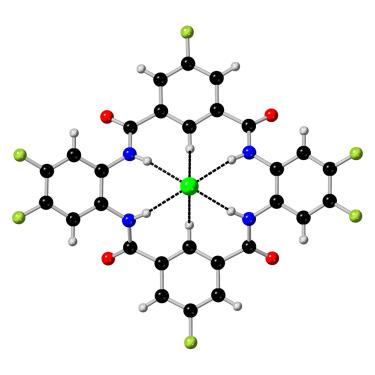

Tetralactam-based anion transporters
Synthetic anion transporters provide a promising avenue to treat diseases such as cystic fibrosis and cancer. Anion binding site preorganization is one aspect of transporter design that can be manipulated to enhance binding. Macrocycles possess preorganized binding cavities, enabling more selective and efficient anion binding and transport. In this study, we build on a macrocyclic tetralactam scaffold by preparing a series of fluorinated and non-fluorinated tetralactam anion transporters. Anion binding and transport assays were used to analyze the substituent effects on scaffold lipophilicity, selectivity, solubility, binding strength, and transport rates. The series was analyzed for the ability to bind and transport Cl− and F− anions across lipid bilayers. Some highly fluorinated tetralactams display extremely high levels of Cl− and F− transport activity, showing record activities in 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS) assays and a Eu(III) probe-based F− transport assay.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


