通过有机催化生产甲烷
IF 49.7
1区 材料科学
Q1 ENERGY & FUELS
引用次数: 0
摘要
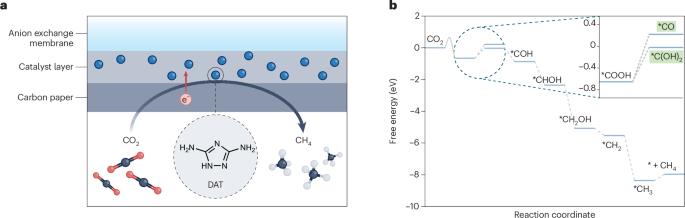
通过电化学方法将二氧化碳还原成燃料和化学品,通常需要使用金属催化剂。现在,一种经有机分子催化剂修饰的碳电极显示出良好的活性和选择性,可通过一种不寻常的途径将二氧化碳电还原成甲烷。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Producing methane through organocatalysis
Electrochemical reduction of carbon dioxide to fuels and chemicals is usually mediated by metal-based catalysts. Now, a carbon electrode modified with an organic molecular catalyst demonstrates promising activity and selectivity for carbon dioxide electroreduction to methane via an unusual pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Energy
Energy-Energy Engineering and Power Technology
CiteScore
75.10
自引率
1.10%
发文量
193
期刊介绍:
Nature Energy is a monthly, online-only journal committed to showcasing the most impactful research on energy, covering everything from its generation and distribution to the societal implications of energy technologies and policies.
With a focus on exploring all facets of the ongoing energy discourse, Nature Energy delves into topics such as energy generation, storage, distribution, management, and the societal impacts of energy technologies and policies. Emphasizing studies that push the boundaries of knowledge and contribute to the development of next-generation solutions, the journal serves as a platform for the exchange of ideas among stakeholders at the forefront of the energy sector.
Maintaining the hallmark standards of the Nature brand, Nature Energy boasts a dedicated team of professional editors, a rigorous peer-review process, meticulous copy-editing and production, rapid publication times, and editorial independence.
In addition to original research articles, Nature Energy also publishes a range of content types, including Comments, Perspectives, Reviews, News & Views, Features, and Correspondence, covering a diverse array of disciplines relevant to the field of energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


