通过统一合成各种磺酰氟发现布氏锥虫抑制剂
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
亲电探针的制备通常使用一套狭窄的强反应工具,这往往会限制其结构和功能的多样性。我们开发了一种统一的磺酰氟骨架多样性合成方法,它依赖于光氧化催化的正己基磺酰氟与氢供体构筑基块之间的脱氢偶联反应。制备了一套 32 种不同的探针,然后针对布氏锥虫进行了筛选。发现其中四种探针具有亚微摩级的抗锥虫活性。利用炔化类似物和广谱荧光膦酸盐工具的化学蛋白质组学方法,对观察到的抗锥虫活性有了深入的了解,这种活性可能源于对多个蛋白质靶点的共价修饰。据设想,以多样性为导向的统一方法可能有助于发现在阐明生物和生物医学机制方面具有价值的亲电探针。亲电生物活性化合物是通过与亲核氨基酸侧链残基反应来识别和调节蛋白质靶点的有用化学工具。在此,作者报告了亲电性磺酰氟探针的模块化合成,并采用化学蛋白质组学方法评估了它们的抗锥虫活性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
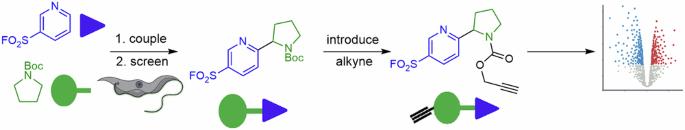
Discovery of Trypanosoma brucei inhibitors enabled by a unified synthesis of diverse sulfonyl fluorides
Sets of electrophilic probes are generally prepared using a narrow toolkit of robust reactions, which tends to limit both their structural and functional diversity. A unified synthesis of skeletally-diverse sulfonyl fluorides was developed that relied upon photoredox-catalysed dehydrogenative couplings between hetaryl sulfonyl fluorides and hydrogen donor building blocks. A set of 32 diverse probes was prepared, and then screened against Trypanosoma brucei. Four of the probes were found to have sub-micromolar anti-trypanosomal activity. A chemical proteomic approach, harnessing an alkynylated analogue and broad-spectrum fluorophosphonate tools, provided insights into the observed anti-trypanosomal activity, which likely stems from covalent modification of multiple protein targets. It is envisaged that the unified diversity-oriented approach may enable the discovery of electrophilic probes that have value in the elucidation of biological and biomedical mechanisms. Electrophilic bioactive compounds are useful chemical tools for identifying and modulating protein targets through reaction with nucleophilic amino acid side chain residues. Here, the authors report a modular synthesis of electrophilic sulfonyl fluoride probes, and evaluate their anti-trypanosomal activity using a chemoproteomics approach
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


