α-三氟甲基苯乙烯与二硫化物的电化学 Giese 反应:高效获取 β-三氟甲基硫醚
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-10-09
DOI:10.1021/acs.joc.4c0168410.1021/acs.joc.4c01684
引用次数: 0
摘要
首次公开了在无金属和温和条件下,α-三氟甲基苯乙烯与二硫化物的电化学吉斯型氢硫化反应。这种方法提供了一种简便的方法,可以从容易获得的底物开始,以中等到良好的产率和较高的官能团耐受性制备 β-三氟甲基硫醚。此外,药物分子的后期修饰和克级合成也显示出实用优势。对照实验和循环伏安法测量揭示了该反应的自由基途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
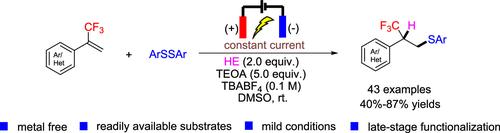
Electrochemical Giese Reaction of α-Trifluoromethylstyrenes with Disulfides: Efficient Access to β-Trifluoromethylated Thioethers
An electrochemical Giese-type hydrothiolation of α-trifluoromethylstyrenes with disulfides is disclosed for the first time under metal-free and mild conditions. This approach provides a facile methodology for β-trifluoromethylated thioethers in moderate to good yields with high functional group tolerance starting from readily available substrates. Additionally, late-stage modification of drug molecules and gram-scale synthesis show practical advantages. The radical pathway of this reaction has been revealed by control experiments and cyclic voltammetry measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


