新型硝基吲哚嗪类化合物的设计、合成、体外和体内杀锥虫功效
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
利什曼病和锥虫病是热带和亚热带国家流行的致命病媒寄生虫病。目前还没有针对这两种疾病的预防疫苗,一旦确诊,只有少数几种临床上可获得的效果较差的药物可用于治疗这些疾病。尽管这些疾病是可以治愈的,但由于病原体出现了对多种药物产生抗药性的菌株,根除和消除这些疾病的工作受到了阻碍。因此,开发新的、有效的和可负担得起的药物变得越来越有必要。近几十年来,包括硝基芳香族化合物、内过氧化物等在内的几种分子支架被尝试用作生成低毒、有效的新型临床抗盘吸虫药物的构件,但至今仍未取得成功。为此,我们通过亲核取代(SN)、酰肼化和希夫碱缩合反应三步法合成了一系列硝基吲哚嗪衍生物,并针对各种利什曼原虫和锥虫物种和菌株进行了评估。研究发现了几种具有体外亚摩尔活性、对哺乳动物细胞无毒性的杀利什曼和杀锥虫药物。其中,硝基呋嗪 11(锝 IC50:0.08 ± 0.03 μM)和硝基噻吩嗪 13(锝 IC50:0.09 ± 0.01 μM)在体内对纳加纳锥虫(Trypanosoma congolense)进行了评估。然而,由于所有小鼠都在感染后 13 天内因高寄生虫血症而死亡,因此在治疗期间只观察到部分疗效。今后的研究将评估抗利什曼病和抗凝集素药物的转化潜力,并进一步确定其分子靶标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
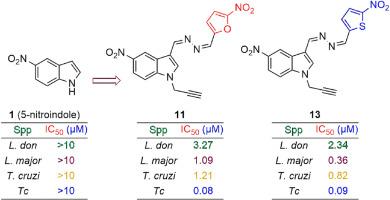
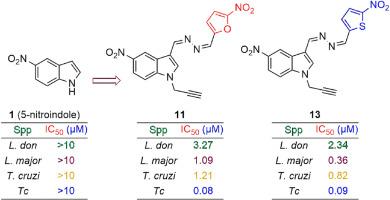
Design, synthesis, in vitro and in vivo trypanosomaticidal efficacy of novel 5-nitroindolylazines
Leishmaniasis and trypanosomiasis rank among lethal vector-borne parasitic diseases that are endemic in tropical and sub-tropical countries. There are currently no preventive vaccines against them, and once diagnosed, a handful of less effective drugs clinically accessible are the only therapeutic options offered to treat these ailments. And although curable, the eradication and elimination of these diseases are hampered by the emergence of multidrug-resistant strains of the causal pathogens. Hence, there is accrued necessity for the development of new, effective, and affordable drugs. In recent decades, several molecular scaffolds, including nitroaromatics, endoperoxides, etc., have been attempted as building blocks to generate new effective clinical antitrypanosomatid agents with low toxicity so far to no avail. In this regard, a series of nitroindolylazine derivatives was synthesised in a three-step process involving nucleophilic substitution (SN), hydrazination and Schiff base condensation reactions, and was evaluated against various Leishmania and Trypanosoma species and strains. Several promising hits portraying leishmanicidal and trypanocidal with in vitro submicromolar activities, and devoid of toxicity on mammalian cells were uncovered. Among these, nitrofurylazine 11 (Tc IC50: 0.08 ± 0.03 μM) and nitrothienylazine 13 (Tc IC50: 0.09 ± 0.01 μM) were evaluated in vivo against Trypanosoma congolense, the causative agent of nagana, which is livestock most virulent trypanosome species in mice-infected preliminary study. However, only partial efficacy was observed as all mice succumbed due to high parasitemia within 13 days post-infection during the treatment. The translational potential of antileishmanial and antichagasic hits, as well as further identification of their molecular targets, will be assessed in future research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


