丰富的界面氧空位和内在氧空位使小型镍/陶瓷纳米晶能够高效甲烷化 CO2
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
较小尺寸的纳米催化剂通常具有丰富的界面空位和固有氧空位,可更好地吸附和活化包括 CO2 在内的小分子,从而提高 CO2 甲烷化的效率。本文采用纳米尺寸较小(约 4.2 纳米)的 Ni/CeO2-S 催化 CO2 甲烷化,与较大纳米尺寸的 Ni/CeO2-L 催化剂相比,其活性显著提高,甚至超过了之前报道的大多数以 CeO2 为载体或以 Ni 为活性金属的催化剂。界面缺陷和内在氧空位的共存增强了 CO2 分子的吸附和活化以及 H 的溢出,从而提高了 CO2 的甲烷化。原位 DRIFTS 显示,Ni/CeO2-S 上几乎只有一种甲酸盐途径可实现高效的 CO2 加氢。这项研究为了解小尺寸纳米支撑催化剂的反应机理提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
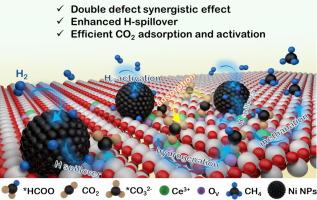
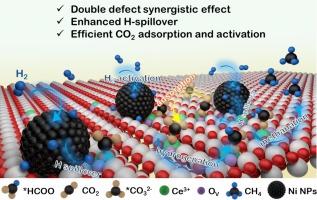
Abundant interfacial and intrinsic oxygen vacancies enabling small nickel/ceria nanocrystal efficient CO2 methanation
Smaller nano-sizes typically result in supported catalysts with abundant interfacial and intrinsic oxygen vacancies for better adsorption and activation of small molecules, including CO2, leading to improved efficiency of CO2 methanation. Here, Ni/CeO2-S with the smaller nano-size of around 4.2 nm is used to catalyze CO2 methanation, which exhibits significantly enhanced activity compared to larger nano-sized Ni/CeO2-L catalyst, even surpassing the most majority of previously reported catalysts using CeO2 as the support or Ni as the active metal. The coexistence of interfacial defects and intrinsic oxygen vacancies allows for enhanced adsorption and activation of CO2 molecules as well as H spillover, resulting in such improved CO2 methanation. In-situ DRIFTS demonstrate a nearly sole Formate pathway on Ni/CeO2-S for efficient CO2 hydrogenation. This research provides valuable insights into the reaction mechanism over a small nanosize supported catalyst.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


