开发膜靶向查尔酮衍生物作为抗耐多药细菌的抗菌剂
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
耐多药病原体引起的感染显著增加,已成为对全球公共卫生的严重威胁。为了开发新的抗菌药物来对抗耐多药细菌,我们设计并合成了一类新型的两亲性查尔酮衍生物,作为抗菌肽拟分子。其中,最有前途的化合物 14b 对革兰氏阳性菌(MICs = 0.5-1 μg/mL)和革兰氏阴性菌(MICs = 1-32 μg/mL)均具有广谱抗菌活性,溶血活性低,膜选择性好。此外,化合物 14b 还具有杀菌作用迅速、产生耐药性的概率低、蛋白水解稳定性高、抑制和破坏细菌生物膜的能力强等特点。进一步的机理研究发现,化合物 14b 具有很强的膜破坏能力,可以通过破坏跨膜电位和提高膜通透性来瓦解细菌细胞膜的完整性,并导致细胞内 ROS 的产生以及 DNA 和蛋白质的泄漏,最终导致细菌死亡。更重要的是,化合物 14b 还在革兰氏阳性菌和革兰氏阴性菌感染的小鼠败血症模型中显示出卓越的体内疗效,这表明它有潜力成为一种抗菌剂来对抗细菌感染。本文章由计算机程序翻译,如有差异,请以英文原文为准。
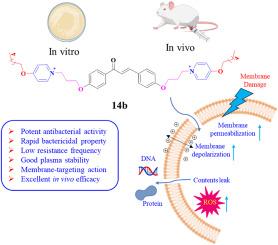
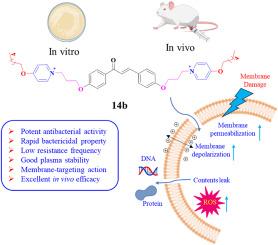
Development of membrane-targeting chalcone derivatives as antibacterial agents against multidrug-resistant bacteria
The striking rise of infections caused by multidrug-resistant pathogens has evolved as a serious threat to public health worldwide. To develop new antibacterials to combat multidrug-resistant bacteria, a novel class of amphiphilic chalcone derivatives serving as antimicrobial peptidomimetics was designed and synthesized. Among them, the most promising compound 14b displayed broad-spectrum antimicrobial activity against both Gram-positive bacteria (MICs = 0.5–1 μg/mL) and Gram-negative bacteria (MICs = 1–32 μg/mL), low hemolytic activity, and good membrane selectivity. Moreover, compound 14b exhibited rapid bactericidal action, a low probability of developing resistance, high proteolytic stability, and strong capabilities of inhibiting and destroying bacterial biofilms. Further mechanism investigations revealed that compound 14b possessed strong membrane-disrupting abilities and could disintegrate the integrity of bacterial cell membranes by destroying transmembrane potential and enhancing membrane permeability, and causing the generation of intracellular ROS and the leakage of DNA and proteins, ultimately leading to bacterial death. More importantly, compound 14b also showed excellent in vivo therapeutic potency in a mouse septicemia model infected by both Gram-positive and Gram-negative bacteria, indicating its potential to be an antibacterial agent to confront bacterial infections.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


