控制钒酸镁和 V2O5 表面对 C1-C3 烷烃初始 C-H 活化反应性的电子和几何特征 - DFT+U 研究
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
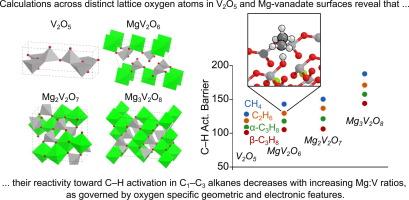
这项研究采用密度泛函理论(DFT+U)计算方法,探讨了 C1-C3 烷烃在 V2O5、MgV2O6(元钒酸盐)、Mg2V2O7(焦钒酸盐)和 Mg3V2O8(正钒酸盐)表面上的初始 C-H 活化。这些材料是烷烃氧化脱氢(ODH)成烯烃的选择性催化剂,与非氧化烷烃脱氢相比,具有实用性和热力学优势。然而,尽管在 ODH 催化过程中,决定烷烃初始 C-H 活化速率的几何和电子特性非常重要,但理论界尚未对这些材料反应性的几何和电子特性进行探讨。在这项研究中,我们探索了 MgxV2Ox+5 (x = 0-3)的十四个低能表面,这些表面暴露了 64 个不同的 O 原子(反应位点)。Mg3V2O8 的 C-H 活化障碍最大,Mg2V2O7 和 MgV2O6 的 C-H 活化障碍较低且相似,V2O5 表面的 C-H 活化障碍最低;这些预测趋势与早期研究中测得的 ODH 反应性一致。当烷烃与结合在单个 V 原子上的 O 原子反应时,反应壁垒最低(平均值),桥接 O 原子的反应壁垒略高,而三重 O 原子的活化壁垒最大。不过,每个子集内部都存在散射现象,这表明 O 原子配位以外的因素对活化势垒也有重要影响。研究发现,空位形成能(VFE)和 O 2p 带能是表面 O 反应性对烷烃活化势垒的微弱描述。相反,氢加成能(HAE)和甲基加成能(MAE)值与烷烃活化势垒有很好的相关性。然而,MAE 的相关性优于 HAE,这是因为 H* 倾向于与附近的表面 O 原子形成 H 键,而这些 H 键在 C-H 活化转变态中不存在,从而导致活化势垒与 HAE 的相关性分散。利用受限轨道 DFT 方法建立了一个理论热化学循环,将 CH3* 的表面还原分解为三个部分:表面变形、轨道定位和键的形成。这些结果让我们深入了解了镁:钒比、表面结构(O 原子配位)和还原性(HAE、MAE)如何影响钒基金属氧化物对烷烃活化的反应性。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Electronic and geometric features controlling the reactivity of Mg-vanadate and V2O5 surfaces toward the initial C–H activation of C1–C3 alkanes – A DFT+U study
This work employs density functional theory (DFT+U) calculations to explore initial C–H activations in C1–C3 alkanes on V2O5, MgV2O6 (meta-vanadate), Mg2V2O7 (pyro-vanadate), and Mg3V2O8 (ortho-vanadate) surfaces. These materials are selective catalysts for the oxidative dehydrogenation (ODH) of alkanes into alkenes, which offers practical and thermodynamic advantages over non-oxidative alkane dehydrogenation. The geometric and electronic properties that govern the reactivity of these materials, however, have not been explored by theory despite their importance in controlling rate determining alkane initial C–H activation during ODH catalysis. In this work, we explore fourteen low-energy surfaces of MgxV2Ox+5 (x = 0–3) exposing 64 distinct O atoms (reaction sites). C–H activation barriers are largest on Mg3V2O8, lower and similar for Mg2V2O7 and MgV2O6, and lowest for V2O5 surfaces; these predicted trends are consistent with measured ODH reactivity in earlier studies. Barriers are lowest (on average) when alkanes react with O atoms bound to a single V atom, with bridging O atoms having slightly higher barriers, and three-fold O atoms having the largest activation barriers. However, there is scattering within each subset indicating that factors beyond O-atom coordination have a significant role in the barriers. Vacancy formation energies (VFE) and the O 2p band energies were found to be weak descriptors of surface O reactivity for alkane activation barriers. Hydrogen addition energy (HAE) and methyl addition energy (MAE) values, in contrast, were found to correlate well with alkane activation barriers. MAE, however, outperforms HAE correlations because of the tendency of H* to form H-bonds with nearby surface O atoms, and those H-bonds are absent in C–H activation transition states causing scatter in the correlation of barriers with HAE. Constrained-orbital DFT methods were used to establish a theoretical thermochemical cycle that decouples surface reduction by CH3* into three components: surface distortion, orbital localization, and bond formation. These results give insights into how Mg:V ratios, surface structure (O-atom coordination), and reducibility (HAE, MAE) impact the reactivity of vanadium-based metal oxides toward alkane activation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


