利用参与级联催化的多酶模拟金单原子纳米酶促进肿瘤凋亡和铁凋亡
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
基于纳米酶的催化疗法可将内源性底物转化为活性氧(ROS),从而诱发肿瘤的氧化应激损伤,因此在癌症治疗中备受关注。然而,纳米酶的有效性受到肿瘤微环境中这些内源性底物有限可用性的阻碍。为解决这一问题,一种新型金基单原子纳米酶(AuSAN)、葡萄糖氧化酶(GOx,G)和乳酸氧化酶(LOx,L)被精心设计成一种高度有序的仿生物复合纳米酶 M/GLB@AuSAN,形成一种相互关联的级联催化反应,将肿瘤碳源催化成 ROS,作为一种持续抗肿瘤策略。负载的 GOx 和 LOx 有氧催化葡萄糖和乳酸产生 H2O2,H2O2 又被 AuSAN 快速转化为-OH、O2--和 O2。生成的 O2 可作为正反馈底物,进一步促进 GOx 和 LOx 介导的有氧催化,显著放大级联催化,从而增强 ROS 的积累。细胞内丰富的 ROS 和稀缺的碳源有效地加剧了蛋白质磷酸化、脂质过氧化和线粒体损伤,最终在体外和体内引发肿瘤凋亡和铁中毒。因此,GOx/LOx/AuSAN 的集成设计为结合多种酶活性、消耗碳源和增强 ROS 生成提供了一种有前途的策略,从而抑制了黑色素瘤的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
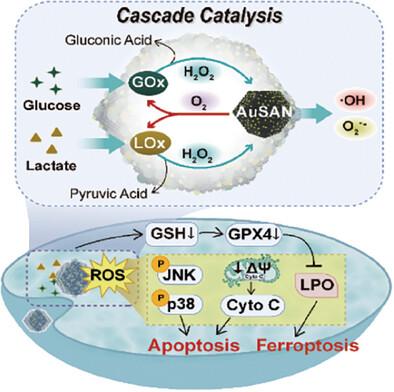
Boosting Tumor Apoptosis and Ferroptosis with Multienzyme Mimetic Au Single-Atom Nanozymes Engaged in Cascade Catalysis
Nanozyme-based catalytic therapy has garnered much attention in cancer treatment for converting endogenous substrates into reactive oxygen species (ROS), which induce oxidative stress damage in tumors. However, the effectiveness of nanozymes is hindered by the limited availability of these endogenous substrates in the tumor microenvironment. To address this, a novel gold-based single-atom nanozyme (AuSAN), glucose oxidase (GOx, G), and lactate oxidase (LOx, L) are meticulously engineered into a highly ordered biomimetic composite nanozyme M/GLB@AuSAN, forming an interconnected cascade catalysis that catalyzes the carbon sources of tumor into ROS as a sustained antitumor strategy. The loaded GOx and LOx aerobically catalyze glucose and lactate to produce H2O2, which is then rapidly converted into ·OH, O2•−, and O2 by AuSAN. The generated O2 serves as a positive feedback substrate for further GOx- and LOx-mediated aerobic catalysis, significantly amplifying cascade catalysis, and thereby enhancing ROS accumulation. The abundant intracellular ROS and scarce carbon sources effectively exacerbate protein phosphorylation, lipid peroxidation, and mitochondrial damage, ultimately provoking tumor apoptosis and ferroptosis in vitro and in vivo. Therefore, the integrated design of GOx/LOx/AuSAN provides a promising strategy to combine multiple enzymatic activities, deplete carbon sources, and enhance ROS production, resulting in the suppression of melanoma progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


