室温下在水中合成 α-羰基-α′-亚磺酰亚磺酰胺
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本研究描述了一种α-羰基-α′-亚磺酰亚磺酰亚胺的高效合成方法,该方法是通过 KIO3 促进芳基硫醇与α-羰基亚磺酰亚胺在水介质中于室温下发生交叉脱氢偶联反应来实现的。α-羰基亚磺酰亚胺和缀有各种官能团的芳基硫醇具有良好的耐受性,并能得到中等至高产率的α-羰基-α′-亚磺酰亚胺衍生物。最后,通过将合成的矢量 3a 转化为其他有价值的化合物,我们证明了这种合成方法的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
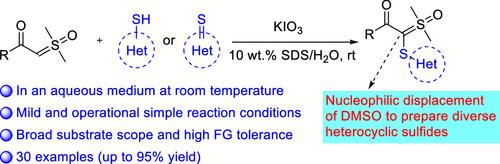
Synthesis of α-Carbonyl-α′-sulfenyl Sulfoxonium Ylides in Water at Room Temperature
An efficient synthesis of α-carbonyl-α′-sulfenyl sulfoxonium ylides through a KIO3-promoted cross-dehydrogenative coupling reaction of aryl thiols and α-carbonyl sulfoxonium ylides in an aqueous medium at room temperature has been described. The α-carbonyl sulfoxonium ylides and aryl thiols adorned with various functional groups were well-tolerated and afforded moderate to high yields of α-carbonyl-α′-sulfenyl sulfoxonium ylide derivatives. Finally, by converting synthesized ylide 3a into other valuable compounds, we demonstrated the practicality of this synthetic method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


