作为 HIV-1 非核苷逆转录酶抑制剂的二芳基嘧啶及相关类似物的研究进展(2019-2023 年)
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
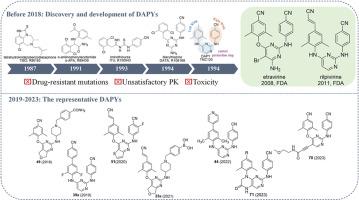
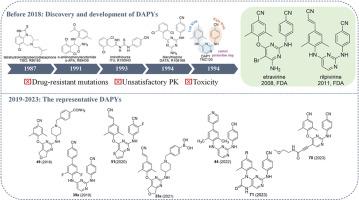
非核苷类逆转录酶抑制剂(NNRTIs)因其独特的抗病毒活性、低毒性和高特异性,已成为高效抗逆转录病毒疗法(HAART)的重要基石。作为第二代 NNRTIs,以依曲韦林和利匹韦林为代表的二乙基嘧啶类(DAPYs)因其高抗病毒效力而受到广泛关注。然而,耐药性突变的快速出现、药代动力学(PK)不理想以及毒性仍然是重大挑战。最近对 DAPY 类似物进行的结构改造主要集中在改善耐药性特征、优化 PK 性能(如半衰期和生物利用度)、通过支架跳跃实现核心结构多样化、改进侧链结构以提高活性和选择性,以及降低毒性和副作用。此外,开发具有广谱抗病毒活性的新型 DAPY 类似物已成为研究的重中之重。本综述全面概述了2019年至2023年DAPY的演变,包括右翼、左翼、中央嘧啶核心和连接体的支架跳跃和结构修饰,为未来开发有效的HIV-1抑制剂提供了宝贵的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Advances in diarylpyrimidines and related analogues as HIV-1 nonnucleoside reverse transcriptase inhibitors (2019–2023)
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) have emerged as a vital cornerstone of highly active antiretroviral therapy (HAART) regimens, owing to their unique antiviral activity, low toxicity and high specificity. Diarylpyrimidines (DAPYs) as the second generation NNRTIs, represented by etravirine and rilpivirine, have attracted extensive attention due to their high anti-HIV potency. However, rapid emergence of resistant mutations, suboptimal pharmacokinetics (PK), and toxicity remain significant challenges. Recent structural modifications of DAPY analogues have focused on improving resistance profiles, optimizing PK properties (such as half-life and bioavailability), diversifying core structures through scaffold hopping, refining side-chain structures to enhance activity and selectivity, and reducing toxicity and side effects. Moreover, developing new DAPY analogues with broad-spectrum antiviral activity has become a key research priority. This review provides a comprehensive overview of the evolution of DAPYs from 2019 to 2023, including scaffold hopping and structural modifications of the right wing, left wing, central pyrimidine core, and linker, affording valuable insights for the future development of effective HIV-1 inhibitors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


