无症状抗核抗体产生的遗传分析
IF 11.4
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
摘要
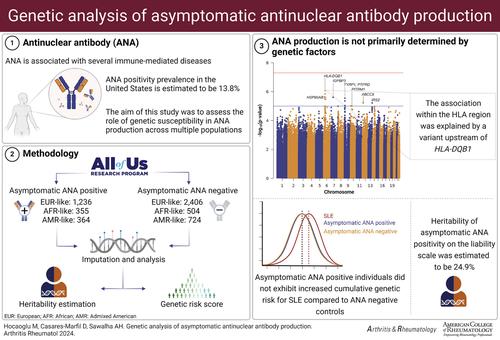
目的:多达 14% 的人群中可检测到抗核抗体 (ANA),而大多数 ANA 患者是无症状的。有关人群中无症状 ANA 阳性的遗传贡献的文献十分有限。本研究旨在对多个人群中的无症状 ANA 阳性进行全基因组关联研究(GWAS)。研究方法纳入 "我们所有人研究计划 "中的无症状 ANA 阳性和阴性个体,选择那些通过免疫荧光法进行 ANA 检测且无自身免疫性疾病证据的个体。研究人员进行了估算,并进行了多人群荟萃分析,其中包括约 600 万个单核苷酸多态性 (SNP)。使用 GCTA 软件估算了基于 SNP 的全基因组遗传率。利用之前报告的全基因组重要基因位点,构建了狼疮累积遗传风险评分。多人群荟萃分析揭示了 8 个不同位点上具有提示性关联的 SNPs(p-value <1×10-5),但未发现具有全基因组意义的位点。HLA-DQB1上游的一个基因变异(rs17211748,P = 1.4×10-6,OR = 0.82,95% CI 0.76-0.89)显示了最显著的相关性。无症状 ANA 阳性的遗传率估计为 24.9%。与 ANA 阴性个体相比,无症状 ANA 阳性个体患红斑狼疮的累积遗传风险并没有增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Genetic analysis of asymptomatic antinuclear antibody production
ObjectiveAntinuclear antibodies (ANA) are detected in up to 14% of the population, and the majority of individuals with ANA are asymptomatic. The literature on the genetic contribution to asymptomatic ANA positivity in the population is limited. In this study, we aimed to perform a genome‐wide association study (GWAS) of asymptomatic ANA positivity in multiple populations.MethodsAsymptomatic ANA positive and negative individuals from the All of Us Research Program were included in this study, selecting those with an ANA test by immunofluorescence and no evidence of autoimmune disease. Imputation was performed and a multi‐population meta‐analysis including approximately 6 million single‐nucleotide polymorphisms (SNPs) was conducted. Genome‐wide SNP based heritability was estimated using the GCTA software. A cumulative genetic risk score for lupus was constructed using previously reported genome‐wide significant loci.Results1,955 asymptomatic ANA positive and 3,634 asymptomatic ANA negative individuals were included across three genetic populations. The multi‐population meta‐analysis revealed SNPs with a suggestive association (p‐value < 1×10‐5 ) across 8 different loci, but no genome‐wide significant loci were identified. A gene variant upstream of HLA‐DQB1 (rs17211748, P = 1.4×10‐6 , OR = 0.82, 95% CI 0.76‐0.89) showed the most significant association. The heritability of asymptomatic ANA positivity was estimated to be 24.9%. Asymptomatic ANA positive individuals did not exhibit increased cumulative genetic risk for lupus compared to ANA negative individuals.ConclusionANA production is not associated with significant genetic risk and is primarily determined by environmental factors.image
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Arthritis & Rheumatology
RHEUMATOLOGY-
CiteScore
20.90
自引率
3.00%
发文量
371
期刊介绍:
Arthritis & Rheumatology is the official journal of the American College of Rheumatology and focuses on the natural history, pathophysiology, treatment, and outcome of rheumatic diseases. It is a peer-reviewed publication that aims to provide the highest quality basic and clinical research in this field. The journal covers a wide range of investigative areas and also includes review articles, editorials, and educational material for researchers and clinicians. Being recognized as a leading research journal in rheumatology, Arthritis & Rheumatology serves the global community of rheumatology investigators and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


