序列长度控制弹性蛋白样多肽中线圈到球状的转变
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
弹性蛋白缩聚物似乎可以确定具有类似液体的特性。然而,最近的一项实验研究表明,它们的聚合状态可能取决于疏水结构域的长度。为了从微观上了解这种行为,我们采用原子模型来评估疏水性弹性蛋白样多肽(ELPs)的构象特性。我们发现,短的弹性蛋白多肽总是保持线圈状构象,而长的弹性蛋白多肽则更喜欢球状构象。前者暂时参与肽内氢键,保持其液体状特性,而后者则形成数百纳秒长的肽内氢键,归因于有序的二级结构图案。我们的研究表明,序列长度可调节弹性蛋白缩合物的材料特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
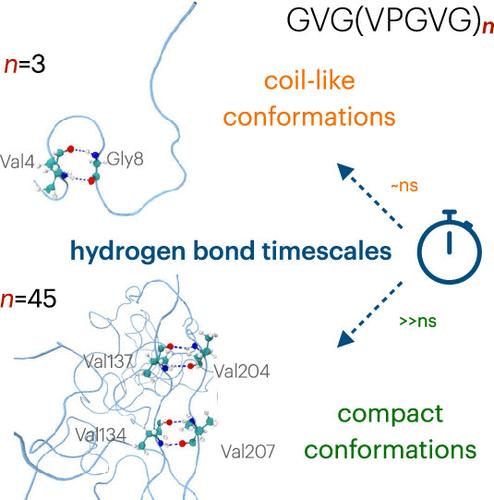
Sequence Length Controls Coil-to-Globule Transition in Elastin-like Polypeptides
It appeared certain that elastin condensates retain liquid-like properties. However, a recent experimental study demonstrated that their aggregate states might depend on the length of hydrophobic domains. To gain microscopic insight into this behavior, we employ atomistic modeling to assess the conformational properties of hydrophobic elastin-like polypeptides (ELPs). We find that short ELPs always remain in coil-like conformations, while the longer ones prefer globule states. While the former engages in intrapeptide hydrogen bonds temporarily, retaining their liquid-like properties, the latter forms hundreds of nanosecond-long intrapeptide hydrogen bonds attributed to ordered secondary structure motifs. Our work demonstrates that the sequence length modulates the material properties of elastin condensates.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


