可清除肾脏的铈复合物和协同抗氧化铁复合物共同用于治疗败血症
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
单核吞噬细胞系统会清除血液中循环的无机纳米材料,这引发了人们对累积金属物种慢性毒性的担忧。因此,临床应用需要更好地了解每种金属在全身注射后的行为。本研究调查了金属-配体相互作用对铈积累的影响,结果表明,只有铈与具有强结合亲和力的多叉螯合剂配位的形式才不会在主要器官中积累。具体来说,与二乙烯三胺五乙酸(DTPA)络合的铈可在体内形成可通过肾脏排出的纳米粒子,以避免铈离子的浸出,而弱配位的铈基纳米材料在遇到生理磷酸盐阴离子时会产生不溶性沉淀物。由铈-DTPA 制成的铈基肾脏可清除纳米粒子(CRNs)与铁-DTPA 作为抗氧化剂配对使用,它们的组合利用芬顿反应协同清除过氧化氢。这不仅降低了被脂多糖激活的巨噬细胞中促炎因子的基因表达,还通过减轻全身炎症反应及其下游的肝、脾和肾组织损伤,提高了脓毒症小鼠的存活率。这项研究表明,CRNs 与铁-DTPA 结合可用作治疗全身性炎症的非累积性纳米药物,从而克服了传统铈纳米粒子疗法的局限性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
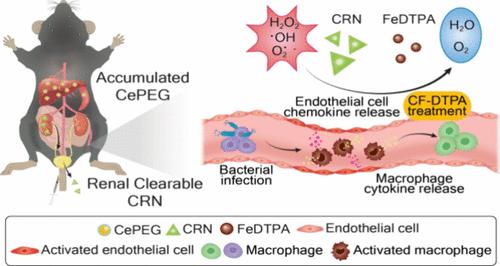
Co-Delivery of Renal Clearable Cerium Complex and Synergistic Antioxidant Iron Complex for Treating Sepsis
The mononuclear phagocytic system clears the circulating inorganic nanomaterials from the bloodstream, which raises concerns about the chronic toxicity of the accumulated metal species. A better understanding of the behavior of each metal after systemic injection is thus required for clinical translations. This study investigates the significance of the metal–ligand interaction on the accumulation of cerium and demonstrates that only the form in which cerium is coordinated to a multidentate chelator with a strong binding affinity does not accumulate in major organs. Specifically, cerium complexed with diethylenetriamine pentaacetic acid (DTPA) forms renally excretable nanoparticles in vivo to circumvent the leaching of cerium ions, whereas weakly coordinated cerium-based nanomaterials produce insoluble precipitates upon encountering physiological phosphate anions. Ceria-based renally clearable nanoparticles (CRNs) derived from cerium–DTPA are utilized as the antioxidant pair with iron–DTPA, in which their combination leverages the Fenton reaction to synergistically scavenge hydrogen peroxide. This reduces the gene expression of pro-inflammatory factors in the macrophages activated with lipopolysaccharide as well as improves the survival rate of septic mice by alleviating the systemic inflammatory response and its downstream tissue injury in the liver, spleen, and kidneys. This study demonstrates that CRNs combined with iron–DTPA can be utilized as nonaccumulative nanomedicines for treating systemic inflammation, thereby overcoming the limitations of conventional ceria nanoparticle-based treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


