再造供体-受体-供体结构的近红外 II 聚合诱导发光发光体,用于饥饿-光热抗肿瘤和抑制肺转移
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
对于开发具有二次近红外(NIR-II)发射的供体-受体-供体(D-A-D)结构聚集诱导发射发光剂(AIEgens)而言,具有强大的电子吸收能力和优异稳定性的电子受体至关重要。虽然 6,7-二苯基-[1,2,5]噻二唑并[3,4-g]喹喔啉(PTQ)和苯并双噻二唑(BBT)被广泛用作 NIR-II 构建模块,但它们在碱性条件下仍存在电子吸收能力有限或化学稳定性不足的问题。本文利用二氟化硼(BFF)受体来构建 NIR-II AIEgen,与 PTQ 和 BBT 衍生的荧光团相比,它在 NIR-II 发射和化学稳定性方面表现出更好的综合性能。TPE-BFF 具有微调的分子内运动和较强的 D-A 相互作用强度,同时表现出较高的摩尔消光系数(ε= 4.31 × 104 M-1cm-1)、较强的近红外-II 发射率(Φ = 0.49%)和光热效应(η = 58.5%)以及较高的稳定性。凭借这些优点,TPE-BFF与抗糖酵解剂2-脱氧葡萄糖(2DG)结合构建的热敏纳米粒子通过调节糖酵解和减少ATP依赖性热休克蛋白,成功地用于成像引导下的光热抗肿瘤肺转移。结合实验结果和理论计算,BFF 被证明是设计多功能 NIR-II AIEgens 的出色电子受体。总之,这项研究为开发生物医学应用中的多功能近红外-II AIEgens提供了一种前景广阔的选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
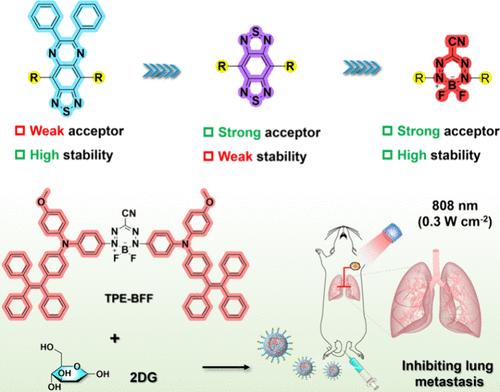
Reengineering of Donor–Acceptor–Donor Structured Near-Infrared II Aggregation-Induced Emission Luminogens for Starving-Photothermal Antitumor and Inhibition of Lung Metastasis
Electron acceptor possessing strong electron-withdrawing ability and exceptional stability is crucial for developing donor–acceptor–donor (D-A-D) structured aggregation-induced emission luminogens (AIEgens) with second near-infrared (NIR-II) emission. Although 6,7-diphenyl-[1,2,5] thiadiazolo [3,4-g] quinoxaline (PTQ) and benzobisthiadiazole (BBT) are widely employed as NIR-II building blocks, they still suffer from limited electron-withdrawing capacity or inadequate chemo-stability under alkaline conditions. Herein, a boron difluoride formazanate (BFF) acceptor is utilized to construct NIR-II AIEgen, which exhibits a better overall performance in terms of NIR-II emission and chemo-stability compared to the PTQ- and BBT-derived fluorophores. With finely tuned intramolecular motions and strong D–A interaction strength, TPE-BFF simultaneously exhibits high molar extinction coefficient (ε= 4.31 × 104 M–1cm–1), strong NIR-II emission (Φ = 0.49%) and photothermal effect (η = 58.5%), as well as high stability. Thanks to these merits, the thermosensitive nanoparticles constructed by integrating TPE-BFF and the antiglycolytic agent 2-deoxy-d-glucose (2DG) are successfully utilized for imaging-guided photothermal antitumor lung metastasis by regulating glycolysis and reducing ATP-dependent heat shock proteins. Combining experimental results and theoretical calculations, BFF proves to be an outstanding electron acceptor for the design of versatile NIR-II AIEgens. Overall, this study offers a promising alternative for developing multifunctional NIR-II AIEgens in biomedical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


